Before we begin, are you sure you understand kinetics? If you need some basic tutorials, you can quickly learn the basics via online games. The most attractive French sites will offer you real money games that you can play and draw the force of bonuses instantly upon you; hurry up and join them today. See, we already start the lesson.
Chemistry in the news – Abstract The oxidation of oxalic acid by the potassium tetrabromoaurate(III) in 0.005≤ [H2C2O4]≤0.07 mol dm-3 is studied by UV spectrophotometry in the temperature range of 293.2 – 313.2 K. Under pseudo-first-order conditions ([H2C2O4]0 ≥10[Au(III)]0), it is first order in [Au(III)] and [H2C2O4]. Both H+and Br– retard the reaction. The reactive species for Au(III) is AuBr3(H2O). A mechanism for this reaction is proposed according to the kinetic study. The rate law derived from the mechanism can explain all the kinetic experiment.
Keywords Potassium tetrabromoaurate(III), oxalic acid, kinetics and mechanism, oxidation.
-
INTRODUCTION
The chemistry of gold(III) is known on the use of its compounds in the treatment of rheumatism (anti-arthritis Au(I) drugs may be activated in vivo to Au(III) metabolites) and Au(III) complexes hold promise as possible anti-tumor agents[1]. For these purpose, a lot of Au(III) complexes have been synthesized.
Au(Ⅲ) has a low spin d8 centre which forms square-planar complexes, such as AuCl4–, AuBr4–. Reduction of Au(III) complexes by various reductants[2,3] has been investigated, such as glycolal[4], sugars [5], o-phenylenediamine[6], sulfite [7], and methionine[8] [9]. However, the kinetics and mechanism for reduction of AuBr4– has little report. The kinetics and mechanism of the reaction of the potassium tetrabromoaurate(III) with oxalic acid is studied in this paper. -
EXPERIMENTAL SECTION
2.1.Chemicals and solutions
KAuBr4(Sigma) used as received, H2C2O4 and NaBr were obtained from Beijing Chemical Reagent Company (Beijing, People’s Republic of China), all other reagents were of A.R. grade. All solutions were prepared with doubly distilled water. The ionic strength was maintained by adding NaClO4 solution and the concentration of H+ was adjusted by adding the HClO4 solution. Solutions of KAuBr4 and H2C2O4 for kinetic study were always freshly prepared before used.
2.2. Apparatus and Kinetics measurements
The rates were measured under pseudo first-order conditions [oxalic acid]0>>[Au(III)]0. 2.00ml of the KAuBr4 solution containing a definite concentration of HClO4 and NaBr was transferred to upper branch of the λ-type tube and 2.00ml of oxalic acid solution with an appropriate concentration and containing a definite concentration of NaClO4 was transferred separately to the lower branch of this tube. After thermal equilibration at the desired temperature in a thermostat, the two solutions were mixed well and immediately transferred into a 1cm thick rectangular quartz cell in a constant temperature cell-holder. The kinetic runs performed on the TU-1900 spectrophotometer (Beijing) fitted with a 501 thermostat (±0.1℃, Shanghai). Monitoring the absorbance of Au(III) complex at 381nm. All other species did not absorb significantly at this wavelength.
2.3. Product analysis
The solution containing 0.10mol/L AuBr4– , 0.10mol/L oxalic acid and 0.10mol/L HClO4 was flushed by nitrogen for at least 10 min. After that 1ml of acrylonitrile was added to the solution. The reaction was carried out at room temperature. After 3 hour, it did not produce any white precipitate indicating that no free radical formed. The absence of radical indicates that a two-electron transfer reaction was happened. 0.10mol/L AuBr4– solution and 0.10 mol/L oxalic acid solution were flushed by N2 for at least 10min, the two solutions were mixed, and then, Ca(OH)2 solution flushed by N2 before used was added into the solution under the N2 protected, a white precipitate was formed indicating the CO2 generated in the reaction[11]. Discover more by reading this post. -
RESULTS AND DISCUSSION
3.1. Evaluation of Pseudo-First Order Rate Constants
Under the conditions of [H2C2O4]0 ?10[KAuBr4]0, Plots of ln(A0– A∞ )/(At-A∞) versus reaction time were linear (Fig 1), suggesting that the reaction is first order in [Au(Ⅲ)], where At and A∞ refer to absorbance at time t and infinity, respectively.
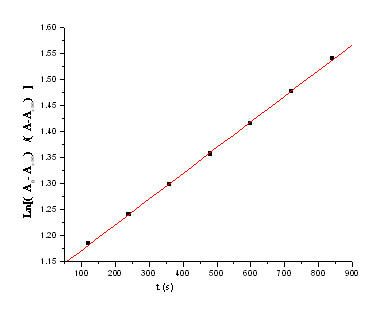
Figure 1 ln[(A0– A∞)/(At-A∞)]versus reaction time t at 40℃.
[Au(III)] = 1×10-4 mol/L; [H2C2O4] = 0.03 mol /L; [Br–] = 0.02 mol/L; [H+]=0.005mol/L; μ =0.43 mol/L
3.2. The dependence of rate on the concentration of H2C2O4
At constant temperature, kobs values increase by increasing the concentration of H2C2O4 while keeping [Au(Ⅲ)],[ H+],[ Br–] and μ constant. Plots of kobs versus [oxalate]tot are linear with a slight intercept indicating the first order with respect to oxalate (Fig 2).

Figure 2 The plots of kobs vs. [oxalate]tot at different temperatures.
Reaction conditions: [Au(III)] = 1.00×10-4 mol/L; [Br–] = 0.02 mol/L;[H+] = 5.00×10-3mol/L;and μ =0.43 mol/L
3.3. The dependence of rate on the concentration of Br–
At constant temperature, kobs values decrease in increasing the concentration of Br– while keeping the [Au (III)], [H2C2O4], [H+] and μ constant. The plot of 1/kobs vs. [Br–] is linear with positive intercept (Figure 3).

Figure 3 The plot of 1/kobs vs. [Br –] at 25℃. Reaction conditions: [Au(III)] = 1.00×10-4mol/L; [H2C2O4]tot = 0.04mol/L; [H+] = 0.01mol/L and μ= 0.60 mol/L.
3.4. The dependence of rate on the concentration of H+
The values of kobs decrease with an increasing in [H+] while keeping [Au(III)], [H2C2O4], [Br–] and μ constant at constant temperature. 1/kobs as a function of [H+] is in Fig 4.
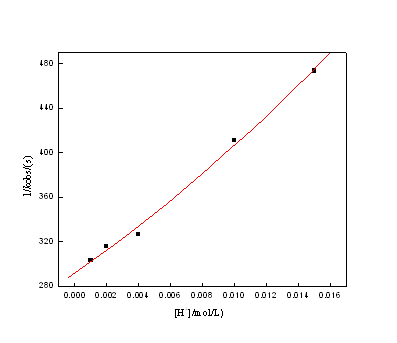
Figure 4 1/kobs as function of [H+] at 25℃. Reaction condition: [Au (III)] = 1.00×10-4 mol/L; [H2C2O4] = 0.03 mol/L; [Br–] =0.01mol/L; μ =0.54 mol/L
3.5. The dependence of rate on the ionic strength
The effect of ionic strength μ was studied while keeping [Au (III)], [H2C2O4], [H+] and [Br –] constant. There is a positive salt effect on the reaction (Table 1).
Table 1 The dependence of kobs on [H+] at 25℃. Reaction condition: [Au (III)] = 1.00×10-4 mol /L; [H2C2O4] = 0.01 mol/ L; [Br–] = 0.02 mol/L; [H+] = 5.00×10-3mol/L
| μ/(mol/L) | 0.15 | 0.25 | 0.45 | 0.65 | 0.85 | 1.00 |
| 104kobs/(s-1) | 2.01 | 2.58 | 3.37 | 4.11 | 4.55 | 4.72 |
4. DISCUSSION OF THE REACTION MECHANISM
4.1. Desirable reaction conditions
Discussion the dissociation of H2C2O4 occurs as equilibriums (1) and (2).The [C2O42-] is negligible under our experimental conditions and the fact that H2C2O4 reacts immeasurably slowly therefore the main reactive species of reducing agent is HC2O4– ion [10] [12].
Because the solution of Au(III) was prepared in strong acid, so we think it exists as HAuBr4. Equilibriums (3) and (4) give good describes of the species of Au(III) under our conditions. The Au(III) exists in experiment conditions as fractions of HAuBr4, AuBr4–, and AuBr3(H2O). From the kinetic study of this reaction system, AuBr3(H2O) as the major reactive species of oxidant[13] [14] [15] has been proposed. It is known that it is a first order with respect to oxalate, so HC2O4– reaction with AuBr3(H2O) directly as the rate determining step.
4.2. Mechanism prevails
Based on the observed kinetic results and analysis, a straightforward reaction mechanism is proposed as below:
![]() (1)
(1)
![]() (2)
(2)
 (3)
(3)
![]() (4)
(4)
![]() (5)
(5)
Where k is the rate constant of the rate determining step Eq(5).
From the equation (3) and (4), AuBr3(H2O) can be expressed as equation(6)
![]() (6)
(6)
Where [Au(III)]tot means the total concentration of Au(III).
From a similar consideration of the equilibrium (1) and (2), and the total concentration of [HC2O4–]tot is given by the equation (7).
![]() (7)
(7)
It is known that Ka1= 0.06 mol/L, Ka2 =6.2×10-5 mol /L from the reference[17]. According to the experimental condition, (Ka1[H?+] + [H?+]2)>>Ka1Ka2 is reasonable. So Eq.(8) was obtained from Eq.(7).
![]() (8)
(8)
The rate law derived from the proposed reaction mechanism can be expressed as:
![]() (5)
(5)
 (6)
(6)
 (7)
(7)
 (8)
(8)
Eq. (6) suggests that the plot of kobs vs. [H2C2O4]tot is straight linear, which through the origin point. Eq. (7) shows that the plot of 1/kobs vs. [Br –] is straight linear with positive intercept, and kobs as a function of [H+] fitted by Eq. (8) is displayed in Fig. 4.
The values of k =0.11 (mol/L)-1·s-1was calculated from the plot of 1/kobs against [H+] at 25℃.
-
CONCLUTION
In this kinetic experiment, a reaction mechanism has been proposed, and the rate law derived from the reaction mechanism can explain all the kinetic phenomena. Through the redox reaction of Au(III) study, it can help the researcher to understand some reaction ability of AuBr4–, when some reactive Au(III) complex were synthesized from the AuBr4–. If the reactive Au(III) complex were used for drug to treat the disease, the redox reaction with some reductants just like GSH must be studied.
REFERENCES
[1] C.F. Shaw, in: H. Schmidbaur (Ed.), Gold: Progress in Chemistry, Biochemistry and Technology, Wiley, New York, 1999.
[2] Florence J. Monlien, Lothar Helm, Inorg. Chim. Acta., 331, 257 (2002).
[3] Lise Drougge, Lars I. Elding, Inorg. Chem, 26, 1073 (1987).
[4] John Wiley Sons Inc. Int. J. Chem. Kinet., 30, 613 (1998).
[5] K.K. Sen Gupta, Carbohydrate. Research., 330, 115 (2001).
[6] ?zlen Altuna, Halide Akbas, Molecular and Biomolecular. Spectroscopy., 66, 499 (2007).
[7] Sen Gupta, K. K; Das, S.; Sen Gupta, S. Transition Met. Chem. 1988,
[8] G. Natile, E. Bordignon, L. Cattalini, Inorg. Chem. 15 (1976) 246�C248.
[9] E. Bordingnon, L. Catalini, G. Natile, A. Scatturin, J.C.S. Chem. Commun. (1973) 878�C879.
[10] B.S. Maritz, R. van Eldik, Inorg Chim Acta, 17 (1976) 21.
[11] Vimal Soni, R.S. Sindal, Raj N. Mehrotra, Inorg. Chim. Acta., 360, 3141 (2007).
[12] V.P. Kazakov, A.I. Matveeva , A.M. Erenburg, B.I. Peshchevitskii, Russ J Inorg Chem (EnglTransl), 10 (1965) 563.
[13] P. van Z. Bekker, W. J. Louw and W. Robb, Inorg. Chim.Acta, 6, 564 (1972).
[14] W.J. Louw and W. Robb, Inorg. Chim. Acta, 3, 303 (1969).
[15] W.J. Louw and W. Robb, Inorg. Chim. Acta, 8, 253 (1974).
[16] V.P. Kazakov and M.V. Konovalova, Russ. J. Znorg. Chem., Z3, 1226 (1968).
[17] Cao xizhang Song tianyou.Inorganic Chemstry (Wu Ji Hua Xue) Wu han university 1994.
