Li Yinke 1,2 , Huang Yun 2 , Hu Qiufen 2,3 , Hhuang Qilin 3 , Yang Guangyu 2,3
( 1 Southwest University, Chongqing 400715; Department of Chemistry, Yunnan Nationalities University, Kunming 650031 ; 2 Department of Chemistry, Yunnan Nationalities University, Kunming 650031; 3 Department of Chemistry, Yuxi Teacher’s College, Yuxi 653100 )
Abstract The solid phase extraction cartridge using multiwalled carbon nanotubes loaded pentathia-15-crown-5 (PT15C5) as sorbent was manufactured, and a solid phase extraction and flame atomic absorption spectrometry method for the determination of gold with this cartridge was studied. The gold can quantificationally adsorbed onto the graphitized carbon black cartridge when they pass the cartridge as pH 3.5 HAc-NaAC buffer medium. Then the gold can eluted from the cartridge with 0.1 mol/L Na2S2O3 solution. By this procedure, the gold in samples can be enriched with high times and most of the interference foreign ions can be separated. The enriched samples were determined by flame atomic absorption spectrometry. Beer’s law is obeyed in the range of 0 – 3.0 mg/mL. This method was applied to the determination of gold in ore and water samples. The relative standard deviations are 2.8 -3.3%, and the recoveries are 88-101%. Results are satisfactory.
Keywords flame atomic absorption spectrometry; gold; solid phase extraction; multiwalled carbon nanotubes; pentathia-15-crown-5
Study on Determination of Gold by Solid-Phase Extraction-Flame Atomic Absorption with Multi-Bi Carbon Nanotubes
Li Yinke1,2 , Huang Yun1 , Hu Qiufen2 , 3 * , Huang Qilin3 , Yang Guangyu2,3 ( 1. College of Resources and Environment, Southwest Agricultural University, Beibei, Chongqing 400715 ; 2. College of Chemistry and Biotechnology, Yunnan University for Nationalities; Kunming 600031 ; 3. Department of Chemistry and Environmental Science, Yuxi Normal University, Yuxi 653100) National Natural Science Foundation of China (50664008) , Natural Science Foundation of Yunnan Province (05E024M) and Yunnan Provincial Young and Middle-aged Academic Leader Training Fund (2004PY01-32)
Abstract A porous polybicarbon nanotube solid-phase extraction column loaded with pentathio -15- crown -5 (PT 15 C 5 ) was prepared, and its solid-phase extraction of gold was studied. Gold-containing samples were extracted at pH 3.5 . After passing through the column in HAc-NaAc buffer medium, the gold can be quantitatively adsorbed on the small column. After enrichment, the gold enriched on the small column can be eluted with 0.1 mol/L sodium thiosulfate, which can realize the separation of low concentration gold in the sample. High multiple enrichment and separation of most interfering ions, the gold in the eluent can be determined by atomic flame atomic absorption spectrophotometry. The method is used for the actual determination of gold in ore and environmental water samples. The relative standard deviation is between 2.8-3.3% , and the standard recovery is between 88-101 %. The results are satisfactory. Key words flame atomic absorption spectrophotometry; gold; solid phase extraction, multi-bi carbon nanotubes, pentathio- 15- crown -5
1 Introduction
There have been many reports on gold determination methods in the literature. Because the content of gold in geological samples and environmental samples is generally low, it is difficult to directly measure gold. Therefore, the problem of pre-enrichment of gold must be solved first. In this regard, solvent extraction, activated carbon adsorption, ion exchange enrichment and foam adsorption are the most commonly used methods, but these methods have long analytical steps and the enrichment factor of gold is not too high [1-4 ] . Solid phase extraction has been widely used in analytical chemistry in recent years because of its advantages of high enrichment multiple, less environmental pollution, less emulsification, and time saving [ 5 – 7] ; currently, reverse-bonded silica gel, polymer , ion exchange resin and other materials are widely used in the sample pretreatment of gold analysis [5,8,9] . Since the discovery of carbon nanotubes in 1991 , it has attracted people’s attention in many research fields and has been widely used in analytical chemistry. Analytical chemistry studies have proved that carbon nanotubes are an excellent reversed-phase solid-phase extraction material. , performance is better than bonded silica gel, graphitized carbon black, polymer and other solid phase extraction materials [10,11] ; but carbon nanotubes used in solid phase extraction separation and enrichment of noble metal elements has not been reported. We have studiedthe separation and enrichment of gold by solid-phase extraction column of multi-walled carbon nanotubes loaded with pentathio- 15- crown -5 (PT 15 C 5 ) , and then determined by flame atomic absorption method. The results show that PT 15 C 5 It can be stably attached to carbon nanotubes, eluted with water medium without loss, and PT 15 C 5 has the ability of molecular recognition, andthe carbon nanotubes loaded with PT 15 C 5 can selectively adsorb gold, and most of the interfering elements are also separated during the high-fold enrichment of solid phase extraction, which makes the method obtain high efficiency . selective. The method is actually used in the determination of gold content in geological samples and water samples, and the results are satisfactory.
-
Experimental part2.1 . Main instruments and reagents
Varian SectrAA 200atomic absorption spectrometer,KY-2gold hollow cathode lamp;Beckman Φ-200acidity meter from American Beckman Company;Multi-bicarbon nanotubes (Aldrich No: 636630 purchased from Aldrich , USA ) were placed in a 50 mL dry beaker, and 10 mL of 5 % PT 15 C 5 ( purchased from Fluka , Switzerland ) acetone solutionwas added under constant stirringEvaporate to dryness, and repeat this process 3 times to prepare multi-walled carbon nanotubes. 10 g of multi-walled carbon nanotubes and 10 g of filter aid (Celite 545) are mixed evenly. Pack the solid phase extraction columnaccording to the method of literature [12,13](0.5 ´ 10 mm) ; the extraction column was washed with 0.1 mol/L sodium thiosulfate, and then washed with HAc-NaAc with a pH of 3.5 , which can be used for gold enrichment.
Gold standard stock solution: 1.0 mg/mL , purchased from the National Standard Material Research and Development Center, and diluted step by step to the standard working solution of the required concentration when used. 2.2 Experimental method Take an appropriate amount of standard sample or sample solution (total gold content < 6.0 mg ), and enrich it by solid-phase extraction with multi-bi carbon nanotube column at a flow rate of 5.0 mL/min. After enrichment, use slightly less than 2.0 mL of 0.05 mol/L sodium thiosulfate solution was used to elute the gold enriched on the small column in the reverse direction. The eluate was collected in a 2.0 mL graduated tube and the volume was accurately adjusted to 2.0 mL for atomic absorption analysis. 2.3 Atomic absorption measurement conditions The analysis line wavelength is 242.8 nm . Hollow cathode lamp current is 4.0 mA . Air flow rate of 6.0 L/min
, the acetylene flow rate is 1.8 L/min . The spectral bandwidth is 0.5 nm . The burner height is 12.5 mm . Measurement Mode: Integral. The measurement time is 10 s .
3 Results and Discussion 3.1 Preparation of carbon nanotubes loaded with PT15C5 Multi-bi carbon nanotubes have no obvious adsorption effect on gold, but multi-bi carbon nanotubes can achieve selective adsorption of gold by attaching reagents that selectively adsorb gold on the surface , PT 15 C 5 has been reported as an extraction agent for gold, and the reagent can be stably attached to multi-bi carbon nanotubes. Due to the cavitation effect of crown ether, the reagent has a molecular recognition effect on gold and can adsorb gold with high selectivity. Therefore, PT 15 C 5 was selected as the loading reagent for multi-bi carbon nanotubes in this experiment. Experiments show that the loading of PT 15 C 5 has an influence on the extraction recovery and extraction capacity of gold, the extraction recovery of gold increases with the increase of loading of PT 15 C 5 , and the loading of PT 15 C 5 exceeds 10%. When the extraction recovery rate of gold began to tend to be complete ( > 96%); and the extraction capacity of the material to gold increased with the increase of PT 15 C 5 loading (see Figure 1); but when the loading exceeded 20%, the material It is easy to agglomerate, and the resistance to pass through the column after being packed into an extraction column is large; therefore, PT 15 C 5 is selected for the experiment
With a loading of 15%, 10.g multi-bi carbon nanotubes were treated three times with 10 mL of 5% PT 15 C 5 acetone solution to prepare uniform PT 15 C 5 loaded carbon nanotubes.
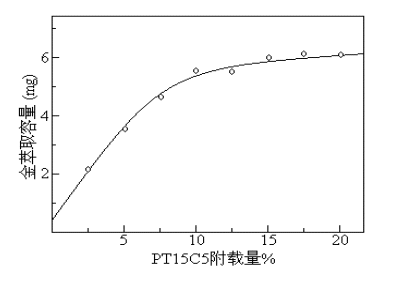
Fig.1 The effect of PT 15 C 5 amount on Au(III) extraction capacity ( 0.2 g carbon nanotubes) Fig.1 The effect PT 15 C 5 amount on Au(III) extraction capacity (0.2 g carbon nanotubes) 3.2 Column The choice of medium tested the effect of the pH value of the column on the gold extraction rate. The results showed that the multi-bi carbon nanotube solid phase extraction column had good adsorption of gold in weakly acidic medium. The gold concentration of 2.0 mg/mL was prepared The solution was passed through the column at different pH values, and the volume of each pass was 50 mL. The results are shown in Figure 2. Experiments show that in the range of pH 2 to 6, gold is well retained on multi-bi carbon nanotubes, and the extraction rate is above 95%. Therefore, the acidity of the sample to pass through the column is 3.5, and HAc with a pH of 3.5 is used. – NaAc buffer solution control. Fig.2 Effect of pH concentration on gold extraction rate Fig.2
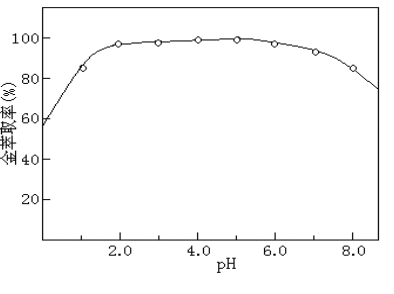
The effect of pH on Au(III) extraction ratio
3.3 Determination of extraction capacity
In order to determine the extraction capacity of the small column (the small column is filled with 0.2 g of PT 15 C 5 loaded carbon nanotubes), a HAc-NaAc buffer medium with a gold concentration of 2.0 mg /mL (pH 3.5) was prepared. The solution was passed through the column, and the results showed that gold began to leak when the column volume exceeded 3000 mL, indicating that the maximum enrichment of gold in the extraction rate column was 6.0 mg (that is, 30 mg Au(III) could be adsorbed per gram of extraction material). Under the conditions of this experiment, the gold content in the sample is only at the microgram level, which will not exceed the extraction capacity of the small column. For gold with a concentration of 0.01 mg /mL and a column volume of 10 L, the recovery rate of gold can reach 94%, which shows that the method is suitable for the enrichment of gold in low-concentration samples, and has a high enrichment factor.
3.4 Selection of the amount of eluent to be used
Table 1 The effect of different eluant on the recovery of gold
| eluant volume | 0.5 mL | 1.0 mL | 1.5 mL | 2.0 mL | 2.5 mL | 3.0 mL | |
| Gold recovery % when using different eluents
The recovery of gold under different eluant% |
HNO3 (0.02 M) | 14.3 | 29.0 | 40.7 | 49.2 | 58.6 | 62.8 |
| HNO3 (0.1 M) | 17.2 | 31.4 | 45.2 | 56.4 | 62.3 | 73.6 | |
| HNO3 (0.5 M) | 24.6 | 42.4 | 60.8 | 68.2 | 81.8 | 86.5 | |
| Thiourea (0.02 M ) |
40.0 | 67.4 | 57.0 | 92.6 | 98.2 | 97.6 | |
| Thiourea ( 0.1 M) |
42.7 | 71.9 | 82.8 | 95.6 | 97.9 | 98.2 | |
| Thiourea ( 0.5 M) |
46.2 | 76.5 | 94.8 | 98.2 | 97.8 | 98.4 | |
| EDTA(0.02 M) | 6.5 | 12.2 | 14.9 | 19.0 | 28.5 | 28.7 | |
| EDTA(0.1 M) | 7.6 | 15.1 | 19.5 | 23.5 | 30.3 | 36.1 | |
| EDTA(0.5 M) | 11.0 | 18.1 | 25.7 | 31.3 | 38.3 | 42.0 | |
| Na 2 S 2 O 3 (0.02 M) | 68.0 | 85.0 | 88.2 | 97.8 | 98.6 | 97.9 | |
| Na 2 S 2 O 3 (0.1 M) | 76.2 | 93.9 | 98.2 | 98.2 | – | – | |
| Na 2 S 2 O 3 (0.5 M) | 83.4 | 96.4 | 97.9 | 99.2 | – | – | |
After the sample is enriched, it can be eluted with eluent. The elution effect of thiourea, nitric acid, EDTA and sodium thiosulfate on gold was tested. The results are shown in Table 1. The experiment shows that when the enrichment amount of gold is 10 mg When using thiourea and sodium thiosulfate solution, the gold can be completely washed off, but the elution effect of sodium thiosulfate is better, so the experiment uses 0.1 mol/L sodium thiosulfate as the eluent, and the dosage is between About 1.5 mL can completely wash off the gold enriched on the small column.

Figure 3 Eluting the cartridge in revised direction after enrichment
After enrichment, turning the small column to reverse direction elution ( Figure 3) can effectively shorten the elution path , reduce the volume of eluent, and increase the enrichment factor. When the gold enriched on the small column is about 10 mg , the forward direction More than 5 mL of eluent is required for elution to completely wash off the gold. If the column is reversed for elution after enrichment, about 1.5 mL of eluent can completely wash off the gold enriched on the column. Down. Therefore, the experiment chose to elute in the opposite direction with slightly less than 2.0 mL of eluent after enrichment .
3.5 Working curve
Under the selected experimental conditions, the Au(III) content in the range of 0.05 ~ 3.0 mg /ml conforms to Beer’s law, and the linear regression is A = 0.274 C ( mg /mL) + 0.0208, r=0.9991 .
3.5 The influence of coexisting ions
For 2.0 mg Au(III) , the relative error is ± 5 %, and the following ions do not interfere ( mg ): NH 4 + , Na + , Cl – , PO 4 3- , SO 4 2- ( 100) ; Ca 2+ , Mg 2+ ,Al 3+ (50);Cr 3+ , Mo( Ⅵ ) , W(VI) , Ti(IV) , Pd 2+ , Sb(III) (40);Pb 2+ , Cd 2+ , Mn 2+ , Ag +,Sn(IV) (20);Ni 2+ , Hg 2+,Pt(IV),Cu 2+ (10);Pd 2+,Rh(III),Ru(III),Ir(IV),Co2+ , Fe 3+ , Zn 2+ (5) ; common elements do not interfere with the determination of gold, and the system has good selectivity.
3.6 Sample analysis and results Take 50 mL
of electroplating wastewater , 500 mL of lake water and river water , acidify with nitric acid and filter with a sand core funnel; then adjust the pH to about 3.5 with sodium acetate , enrich through the column according to the experimental method and select atoms Determination of absorption conditions, the results are shown in Table 2 . Table 2 Sample analysis and results Table 2 Determination results of Samples
| Samples | Measured value (Found) | ICP-MS法 (ICP-MS method) |
RSD% (n=5) |
Standard recovery (Recovery) % (n=5, plus Au(III) 0.2 m g) |
| Electroplating wastewater (Wastewater) |
68.8 (mg/L) | 66.9 (mg/L) | 3.1 | 93 – 96 |
| River water | 8.42 (mg/L) | 8.39 (mg/L) | 3.2 | 88 – 95 |
| Lake water | 3.43 (mg/L) | 3.26 (mg/L) | 3.3 | 90 – 94 |
| Gold Ore (Ore) | 3.24 (mg/g) | 3.38 (mg/g) | 2.8 | 92- 101 |
Depending on the gold content of the ore sample, take 1 to 10 g of the sample as appropriate and place it in a magnetic dish, roast it at 600 to 700 °C for 2 to 4 hours , transfer it to a 400 mL beaker after cooling , soak it with water, and add 50 mL of concentrated hydrochloric acid to decompose the sample 20 min , then add 20 mL of concentrated nitric acid to decompose, heat and evaporate to near dryness on an electric heating plate, heat 10 mL of 1% animal glue solution while hot, stir vigorously, then add 100 mL of 5% hydrochloric acid, stir, clarify, and filter . The filtrate was adjusted to a pH of about 3.5 with sodium acetate , enriched by column according to the experimental method, and determined according to the selected atomic absorption conditions. The results are shown in Table -1 .
4 Conclusions
(1)In this experimentPT 15 C 5 loaded multi-bi carbon nanotubes were selected as the solid phase extraction material. Carbon nanotubes can be used at anypHand special temperature, and their application range and durability are much higher than other reverse materials. ; and the multi-bi carbon nanotubes can be stably loaded withPT 15 C 5 , the reagents attached will not be lost whenthe water medium(pH2-12)PT 15 C 5 can be effective for gold Molecular recognition ability, highly selective adsorption of gold, and separation of most interfering elements while enriching gold, make the method have high selectivity. (2)The particle size of multi-bi carbon nanotubes is small, and after being loaded withPT 15 C 5 , the exchange and elution between gold and the extraction material can be carried out more quickly and completely, and high-fold enrichment of gold can be achieved by using this solid-phase extraction. (3)A sieve plate-type solid phase extraction column is designed. The small column can also be eluted in the opposite direction, shortening the elution path to reduce the volume of the eluent and increase the enrichment factor; it is also convenient for batch samples to be processed at the same time. In conclusion, the establishment of this method provides a method for the determination of trace gold in complex samples.
REFERENCES
[1] Luo Fuzhong . Current status and development trend of domestic gold analysis methods . 1991 , 11(8) : 325-330
[2] Zhu Liqin, Li Youmin . Journal of Central South University of Technology . 2003 , 34(2) : 156-157
[3] Yang Zuoge, He Mingzhong . Foreign Analytical Instrument Technology and Application . 2000 , 22(3) : 64-66
[4] Zhao JS, Li JS, Huang ZJ, Wei QY, Chen J, Yang GY. Title: Solid phase extraction and spectrophotometric determination of gold. Indian journal of chemistry (A). 2006, 45(7): 1651-1654
[5] Zhang Haixia . Analytical Chemistry . 2001 , 28 (9): 1172~ 1180
[6] Hu QF, Chen XB, Yang XJ, Huang ZJ, Chen J, Yang GY. Analytical sciences. 2006, 22 (4): 627-630. [7] Dong XC, Han Y, Hu QF, Chen J
, Yang GY. Journal of the brazilian chemical society. 2006, 17(1): 189-193.
[8] Chen ZY, Huang ZJ, Chen J, Yin JY, Su QD, Yang GY. Analytical letters 2006, 39(3) : 579-587
[9] Camel V. Spectrochimica Acta Part B, 2003, 58(7): 1177-1233 [
10] Zhang Changming . Chromatography, 1992 , 10(2):78-81
[11] Hennion M C. Journal of Chromatography A, 2000, 885(1): 73-95
[12] Zhong Yihui, Huang Qilin, Zhang Xin, Huang Zhangjie, Hu Qiufen, Yang Guangyu . 2 Spectroscopy and Spectral Analysis, 2007 , 27(2) : 360-363
[ 13] Zhou Shiping, Duan Changqun, Liu Hongcheng, Hu Qiufen.Spectroscopy and Spectral Analysis . 200 , 25(10) : 1667-1670
