Wei Yanlin, Lin Jinming
(Research Center for Eco-Environmental Science, Chinese Academy of Science, P.O. Box 2871,
Beijing 100085, China)
Received Nov. 2, 2003; The Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Abstract In this paper, determination methods of total carbon concentration (TC), including total inorganic carbon (TIC) and total organic carbon (TOC) were summarized. TIC determination in natural water samples is essentially vital, especially in sea water samples, for studying the present and future global CO2 air-sea flux. Flow injection analysis (FIA) coupled with a gas diffusion unit (GDU) is proved to be simple, sensitive, rapid and easy to operate for TIC determination. TOC determination is one of the most important works for acquiring knowledge about water and wastewater quality. TOC analysis procedures include two steps, firstly converting the component of organic matter to CO2 and then detecting of the CO2 or carbonate ion in CO2 absorbing solution.
Keywords Total carbon concentration; Total organic carbon; Total inorganic carbon
1 INTRODUCTION
Items of carbon analysis in water sample include total carbon concentration (TC), inorganic carbon (IC), also known as total inorganic carbon (TIC), total organic carbon (TOC), non-purgeable organic carbon (NPOC), purgeable organic carbon (POC), dissolved organic carbon (DOC) and volatile organic compound (VOC) or volatile organic compounds (VOCs). Among those, TC including TIC and TOC are more important than others.
Determination of TC is an important work in environmental science and engineering. It is possible to classify the different fractions of carbon according to the physical or chemical properties of the compounds present in the sample. Thus, one can find the so-called IC, or TIC, which corresponds to the sum of dissolved carbon dioxide, bicarbonate and carbonate, and TOC, which is defined as the amount of carbon covalently bonded as organic compounds present in the sample [1,2]. TOC is obtained from the difference between TC and IC, i.e. TOC = TC-IC, or the sum of NPOC or POC, i.e. TOC = NPOC + POC. Therefore, the precision might be significantly improved by determining these parameters in a direct fashion.
2 TIC
Carbon dioxide (CO2) can dissolve in environmental waters, and is kept at the equilibrium between the atmosphere and the aquatic environment. The exchange of CO2 across the air/water is an important parameter for understanding the processes related to the carbon cycle within the aquatic environment. In contrast to the freshwater systems, the oceans are a huge reservoir of CO2, and can act as both a source and a sink of CO2, depending upon the region, season, and the occurrence of episodic events. Widespread mapping of the partial pressure of CO2 and TIC of seawater is essentially vital for determining the present and future global CO2 air-sea flux. Several methods for the determination of TIC in aqueous solutions have been reported.
Commonly, the water sample is firstly acidified with sulfuric acid and give CO2 gas, then measuring this CO2 with infrared spectrophotometer, gas chromatography or other methods [3], or absorbing it with Ba(OH)2 solution and then followed by titration. For saving time and sensitive determination of CO2, various kinds of methods were proposed.
CO2 sensors based on solid electrolyte were widely used in practice for the monitoring of CO2 in industrial waste gas [4,5]. These solid electrolyte sensors have wide dynamic ranges and their response times are in the ranges of 8 to 40s. However, they always have to be worked at high temperature (600-1100 K) and their response is sensitive to temperature changes [6-8]. They are not adapted for the determination of CO2 in water samples at room temperature. Fiber optic sefnsors for CO2 have been extensively studied [9,10]. However, their sensitivity and precision are not enough for their application to all water samples.
Chemiluminescence is another method but less used for the determination of CO2. Lan et al. [11,12] found that luminol- cobalt(II) phthalocyanine chmiluminescence could be enhanced by CO2 in the absence of added oxidant. Based on this property, CO2 was determined from about 50 ppm to 800 ppm. This system gives a fast response and is capable of reaching detection limits down to 1.5 ppm when working at steady state conditions with the gaseous sample being continuously pumped. However, the system employs an expensive reagent mixture, the signal is pH-dependent and the chemiluminecence detection system is somewhat complex [11].
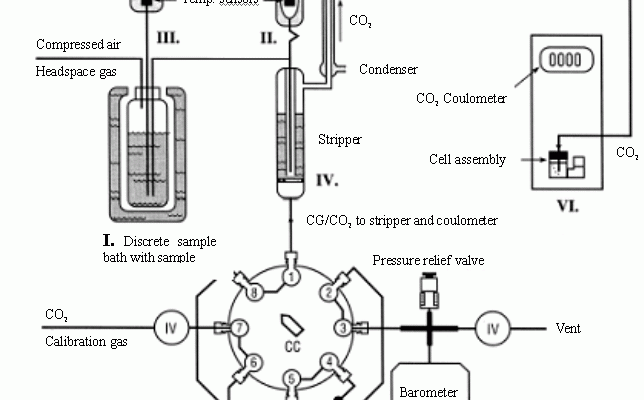
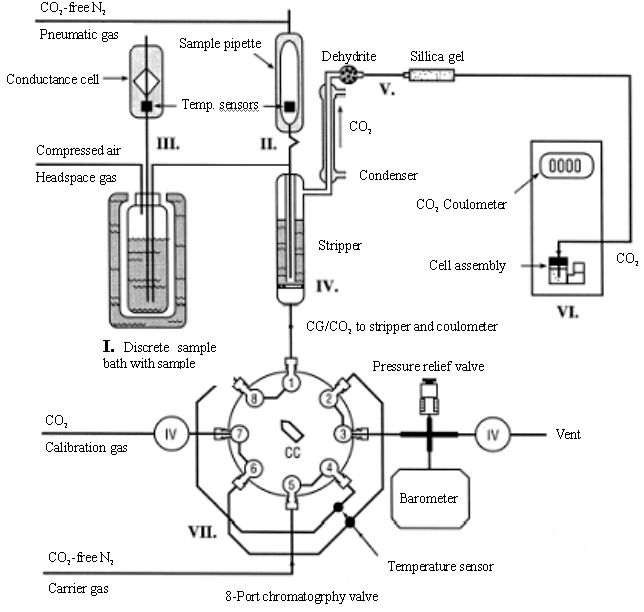
The single operator multiparameter metabolic analyzer (SOMMA) proposed by Johnson et al. [13,14] has been widely used in practical determination of TIC in sea water. Descriptions of the SOMMA-coulometer system and its calibration can be found in the works of Johnson from 1985 to 1993. A schematic diagram of the SOMMA is shown in Fig. 1 , briefly, seawater fills an automated sample pipette. The contents of the pipette are pneumatically injected into a stripping chamber containing approximately 1.2 cm of 8.5% v/v phosphoric acid, and the resultant CO2 is extracted, dried, and coulometrically titrated. Calibration is performed by titrating known masses of pure CO2 and checked by analyzing certified reference material (CRM). The system, however, is very big, complicated and difficult to operate [15].
Gas permeation methods coupled with flow injection analysis (FIA) have been proved to be simple, sensitive, rapid and easy to operate. In the methods, the changes of physicochemical property of the CO2 receptor, which are caused by permeated CO2, can be detected by potentiometry[16], spectrophotometry [17-21] and conductometry[21,22].
The flow analysis used for the determination of TIC with membrane separation was first reported by Carlson [23]. The method was based on the transfer of CO2 by diffusion through silicone-rubber hollow fibres into a flowing stream of deionized water, followed by electrical conductivity detection. Jardim et al. [24] used a FIA system, which was proposed by Pasquini and de Faria [25] for the measurement of ammonia based on gas diffusion through a PTFE membrane and measurement in a conductance flow cell, to monitor aqueous CO2 production from Escherichia coli in toxicity tests. More recently, other authors have also published articles based on the same system for the determination of TIC [26].
Kuban and Dasgupta [21] compared the conductometry with spectrophotometry, and found that the conductometric procedure with a weak alkaline receptor solution provided better day-to-day reproducibility, sensitivity and detection limits.
Aoki et al. [22] improved a conductometric method based on a continuous flow reported by Carlson [23]; the limit of detection corresponding to the signal-to-noise ratio of 3 was 1.0 x 10-5 M. Motomizu et al. reported a gas-diffusion/FIA (GD/FIA) for carbonate [17-19] and ammonium [19,20], and recently Higuchi et al. developed a stable GD apparatus for ammonia determination [27]. Futher evolution on this GD unit (GDU) was made and used for the determination of TIC in various water samples , especially in the purified water samples [28,29].
For improving the sensitivity, new indicators were sythesised and used for the determination of TIC in water samples [28]. A new indicator named 4-(2’,4’-dinitrophenylazo)-1- naphthol-5- sulfonic acid is much better than that of cresol red, which is the best one in commercial available indicators. The limit of detection (LOD) corresponding to a signal-to-noise ratio of three was 1×10-6 M.
For continuous in-situ analysis of TIC in natural water samples, Wei et al. [30] proposed a reversed-flow injection analysis (rFIA) method based on the improved GDU and a portable FIA system [31].
3 TOC
TOC is one of the most important parameters for acquiring knowledge about water and wastewater quality because it concerns theoretically all organic compounds [32]. It is shown that TOC becomes more and more important in the environmental protection areas than other parameters such as biochemical oxygen demand (BOD) and chemical oxygen demand (COD), because TOC is a more suitable and direct expression of the organic pollutants than BOD or COD. The assessment is based on two main reasons: (i) TOC is directly correlated with the carbon concentration, irrespective of the oxidation state of the organic compounds, and (ii) theoretically, TOC is not influenced by the presence of some inorganic reducing agents [33].
TOC has been successfully employed as a general pollution indicator for volatile and non-volatile organic compounds. There are many kinds of commercial available TOC analyzers, for example, TOC-4100 of Shimadzu Corporation, the basic analysis principles are all the same. The typical flow diagram of TOC determination is as shown in Fig. 2. Analysis procedures include, normally, two critical steps [34].
The first one refers to the conversion of all organical bounded carbon to a simple molecular form, which can be measured quantitatively. As a rule, the most usual method for this conversion is the oxidation of organic carbon to carbon dioxide, which can be done by high temperature combustion, chemical or radiative oxidation, such as thermal combution [35,36], pyrolitic methods [37], persulfate oxidation [36,38] and photodecomposition by ultraviolet radiation [39-43].
In these methods, called high-temperature combustion methods, the oxidation of the carbon-containing species is normally carried out in vapor phase in the presence of a catalyst at temperatures ranging from 700 to 900 °C. The main analytical problem in the TOC determination by high temperature combustion methods arises from the difficulty in controlling the temperature in the oxidation step, leading to deterioration in the precision of the results. Additional pitfalls of the high temperature combustion methods include [44] (i) appearance of long memory effects, (ii) capillary blocking when working with high-salt-content solutions or with samples containing suspended solid matter, (iii) high background levels due to carbon release from the catalyst and some other parts of the system, (iv) sometimes the oxidation yield is too low (e.g., 82% for sulfathiazole), and (v) mechanical problems caused by the sudden expansion of the carrier gas stream as it enters the high temperature column [45].
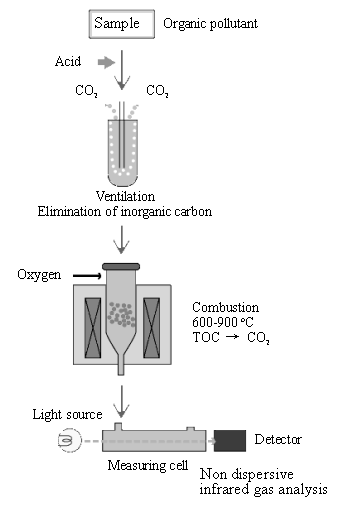
Fig. 2 Flow diagram of TOC determination
Photodecomposition cannot be used for noncombustible compounds and the wet oxidation is useful only for nonvolatile organic carbon, and the pyrolitic methods are restricted to samples with low organic carbon content[46].
The second step is concerning to the detection of produced CO2 during the oxidation of the organic compounds. A carrier gas stream (e.g., oxygen [36], helium [47], argon [45], air or nidrogen [48], and so on) is used to drive the CO2 toward the detector. The CO2 may be determined by means of nondispersive infrared spectrometry [49], near-infrared spectrometry [50], thermal conductivity [35], or indirectly, by acid/base titration of a CO2-absorbing solution [51], and also, by gravimetry [52] or ionic chromatography [36], include the use of a hydrogen-flame ionization detector [47].
A procedure for the determination of TOC is proposed based on the development of a microscale system for the wet decomposition and the conversion of the organic compounds to CO2. The construction and fitness of a microreactor for gas–liquid transfer were proved to be suitable for FI and the turbidimetric determination of the generated CO2 [46].
Plasma spectrometric techniques have been hardly applied to carbon determination [56]. An inductively coupled plasma atomic emission spectrometer system was proposed [45] for the direct determination of TOC and TIC.
To determine the TOC, the TIC must be removed from the sample. To this end, several methods have been proposed [53,54], the most common one being the addition of acid to the sample, to transform inorganic carbon into CO2 and the use of a sparging/carrier gas stream to remove and drive the carbon dioxide toward the measurement zone [36]. Alternatively, for the TIC determination, the sample can be injected into a separated reaction chamber packed with phosphoric acid-coated quartz beads [55]. TIC can also be determined through the use of a semiautomated coulometric method. In this case, the calibration is performed from standards containing known concentrations of bicarbonate [43].
-
PERSPECTIVES
Monitoring TOC in environmental water is very important. In a recent China-Japan environmental technology symposium, it is said that, the standard of COD in tap water will be replaced by the standard of TOC on 1 April 2005 in Japan. There are so many methods for monitoring TOC have been developed as reviewed above beside the standards, but all those methods for determination of carbon concentration in water samples, even in air or solid samples are related with each other. We can select a proper determination method and instrument from commercial available analyzers or develop a new method and a new instrument according to the analytical aims. However, a small portable instrument for continuous, stable and automatic on-site monitoring of TIC and TOC is strongly desired, such as portable FIA[30-31]and optic fiber sensors. Optic fiber sensors may become the perfect sensors for environmental monitoring and analysis. It is very suitable for automatic on-site environmental monitoring and analysis, especially for data network environmental monitoring system. The key point for making optic fiber sensors is to make a sensitive and selective micro membrane for the monitoring target with different materials, such as microorganism, luciferase or molecularly imprinted polymers etc.
REFERENCES
[1] Harrison J F, McGowan W. WAQ Glossary of Terms. 3rd Edtion. Lisle, IL: Water Quality Association, 1997.
[2] Maestre S E, Mora J, Hernandis V et al. Anal. Chem., 2003, 75: 111.
[3] JIS K0304, Method for determination of CO2 in air. Tokyo: Japanese Industrial Standards Committee, 1996.
[4] Wang L, Kumar R V. Solid State Ionics, 2003, 158: 309.
[5] Qiua F, Zhua Q, Yanga X et al.Sensors and Actuators B, 2003, 93: 237.
[6] Ishihara T, Kometani K, Hashida M et al. J. Electrochem. Soc., 1992, 139: 2881.
[7] Miura N, Yao N, Shimizu Y et al. J. Electrochem. Soc., 1992, 139: 1384.
[8] Imanaka N, Murada T, Kawasato T et al. Chem. Lett., 1992, 21:103.
[9] Munkholm C, R.Walt D, Milanovichi F P. Talanta, 1988, 35: 109.
[10] Wolfbesis O S, Weis L J, Leiner M J P et al. Anal.Chem., 1988, 60: 2028.
[11] Lan Z-H, Mottola H A. Anal. Chim. Acta, 1996, 329: 305.
[12] Lan Z-H, Mottola H A. Analyst, 1996, 121: 211.
[13] Johnson K M, King A E, Sieburth J M. Mar. Chem., 1985, 16: 61.
[14] Johnson K M, Dickson A G., Eischeid G et al. Marine Chemistry, 1998, 63: 21.
[15] Ishii M. Bunseki, 2000, 311: 658.
[16] Linares P, Lugue de Castro M D, Valcarcel M. Anal. Chim. Acta, 1985, 225: 443.
[17] Motomizu S, Toei K, Kuwaki T et al. Anal.Chem., 1987, 59: 2930.
[18] Kuwki T, Toei K, Akiba M et al. Bunseki Kagaku, 1987, 36: T132.
[19] Sanada M, Oshima M, Motomizu S. Bunseki Kagaku, 1993, 42: T123.
[20] Kuwaki T, Akiba M, Oshima M et al. Bunseki Kagaku, 1987, 36: T81.
[21] Kuban V, Dasgupta P K. Talanta, 1993, 40: 831.
[22] Aoki T, Fujimura Y, Oka Y et al. Anal. Chim. Acta, 1993, 284: 167.
[23] Carlson R M. Anal. Chem., 1978, 50: 1528.
[24] Jardim W F, Pasquini C, Guimaraes J R et al. Water Res., 1990, 24: 351.
[25] Pasquini C, de Faria L C. Anal. Chim. Acta, 1987, 193: 19.
[26] Hall P O J, Aller R C. Limnol. Oceanogr., 1992, 37: 1113.
[27] Higuchi K, Inoue A, Tsuboi T et al. Bunseki Kagaku, 1999, 48: 253.
[28] Oshima M, Wei Y, Yamamoto M et al. Anal. Sci., 2001, 17: 1285.
[29] Wei Y, Oshima M, Motomizu S. Analyst, 2002, 127: 424.
[30] Wei Y, Oshima M, Takayanagi T et al. J. Flow Injection Anal., 2001, 18: 156.
[31] Ma L, Oshima M, Takayanagi T et al. J. Flow Injection Anal., 2000, 17: 188.
[32] Thomas O, El Khorassani H, Touraud E et al. Talanta, 1999, 50: 743.
[33] Stoll M H C, Bakker K, Nobbe G H et al. Anal. Chem., 2001, 73: 4111.
[34] APHA-AWWA — Standard methods for the examination of water and wastewater. 19th Edition. American Public Health Association, 1995.
[35] Gács I, Payer K. Anal. Chim. Acta, 1989, 220: 1.
[36] Fung Y S, Wu Z, Dao K L. Anal. Chem. 1996, 68: 2186.
[37] Pickering W F. Environmental Science and Technology Series. Vol. 2. New York: Marcel Dekker, 1977.
[38] McKenna J H, Doering P H. Mar. Chem., 1995, 48: 109.
[39] Matthews R W, Abdullah M, Low G K. Anal. Chim. Acta, 1990, 233: 171.
[40] Abdullah M I, Eek E. Wat. Res. 1996, 30: 1813.
[41] Goulden P D, Brooksbank P. Anal. Chem. 1975, 47: 1943.
[42] Van Steenderen R A, Lin J S. Anal. Chem. 1981, 53: 2157.
[43] Johnson K M, Wills K D, Butler D B et al. Mar. Chem. 1993, 44: 167.
[44] Spyres G, Nimmo M, Worsfold P J et al. Trends Anal. Chem. 2000, 19: 498.
[45] Maestre S E, Mora J, Hemandis V et al. Anal. Chem. 2003, 75: 111.
[46] Paniz J N G, Flores E M M, Dressler V L et al. Anal. Chim. Acta, 2001, 445: 139.
[47] Dobbs R A, Wise R H, Dean R B. Anal. Chem. 1967, 39: 1255.
[48] JIS K0805, Continuous Total Organic Carbon Analyzer. Tokyo: Japanese Industrial Standards Committee, 1993.
[49] APHA-AWWA — Standard methods for the examination of water and wastewater. 19th Edition. American Public Health Association, 1995.
[50] Thomas O, Khorassani H El, Touraud E et al. Talanta, 1999, 50: 743.
[51] Nelson D W, Sommers L E. Methods of Soil Analysis. Part 2, Madison, Wis., LISA: American Society of Agronomy, Inc., 1986, p. 1349.
[52] Allinson L E, Bollen W B, Moodie C D. Methods of Soil Analysis. Part 2, Madison, Wis., LISA: American Society of Agronomy, Inc., 1986, p. 1346.
[53] Kubala S W, Tilotta D C, Busch M A. Anal. Chem. 1989, 61: 1841.
[54] Huber S A, Frimmel F H. Anal. Chem. 1991, 63: 2122.
[55] Sugimura Y, Suzuki Y. Mar. Chem. 1988, 24: 105.
[56] Wu M, Carnahan J W. J. Anal. At. Spectrom. 1992, 7: 1249.