In this Chemistry Magazine,you have chemistry related news about a molecularly imprinted polymers (MIP) for sarafloxacin was prepared by the use of itaconic acid as the functional monomer and ethylene glycol dimethacrylate as the crosslinking monomer. But we will also show you the funny side of chemistry, as we earlier discussed the games we stumbled on. Why don’t you take a free test at the casino laboratory by discovering the best casino bonus chips at freecasinogameschips.com? You will find many games that will satisfy your scientific potential. Not let’s go back to the real topic, shall we? To improve selectivity, cobalt(II) ions was used to form metal-template complex, which provided the specific binding sites similar to metal-chelate antibody. The influences of different anions and cations on the recognition performance of the MIP were investigated in protic solvent. Due to the specific structure of the complex of cobalt(II)-sarafloxacin, it increased …
Effects of cation-anion on recognition characteristics of sarafloxacin -Co(II) complex imprinted polymer in aqueous media the other tested compoun

Cure Kinetics of Epoxy Resin/MP/Anhydride/Accelerant Flame Retardant Composite
Before we start, I like to mention my latest discovery on the internet – chemical slot games. I stumbled on them on accident while researching something and I was so impressed and entertained after playing them for a couple of hours that I had to share it with you. Just visit this site to get a free no deposit bonus and play them without risking money. And now let’s talk about Cure Kinetics.
Abstract Cure kinetics of epoxy resin E51/MP/methyltetrahydrophthalic anhydride (MeTHPA)/ 2-ethyl-4-methyl-imidazole (2,4-EMI) flame retardant composite were studied using the dynamical tortional vibration method. The influence of MP loadings on The cure showed has improved studied were well. The results showed that the rate of cure reactions depended distinctly on the cure temperature. At low MP loadings, the gelation time t g for the systems did Not change obviously while at higher MP loading (higher than 20 phr) t …
Kinetics and mechanism of the oxidation of oxalic acid by potassium yetrabromoaurate (III)

Before we begin, are you sure you understand kinetics? If you need some basic tutorials, you can quickly learn the basics via online games. The most attractive French sites will offer you real money games that you can play and draw the force of bonuses instantly upon you; hurry up and join them today. See, we already start the lesson.
Chemistry in the news – Abstract The oxidation of oxalic acid by the potassium tetrabromoaurate(III) in 0.005≤ [H2C2O4]≤0.07 mol dm-3 is studied by UV spectrophotometry in the temperature range of 293.2 – 313.2 K. Under pseudo-first-order conditions ([H2C2O4]0 ≥10[Au(III)]0), it is first order in [Au(III)] and [H2C2O4]. Both H+and Br– retard the reaction. The reactive species for Au(III) is AuBr3(H2O). …
Preparation and characterization of high molecular weight poly(L-lactic acid) by chain extending

Abstract Poly(L-lactic acid) (PLLA) of high molecular weight was prepared by chain extending reaction in a micro-compounder. Phosphites were used as chain extenders to increase the molecular weight of the PLLA prepolymer that was prepared by bulk polycondensation of L-lactic acid. Chemistry Magazine Online shows the effects of the amount of the phosphite, temperature and the screw speed were studied on the torque of PLLA melt in mixing. There’s also another thing that this magazine provides, something you can do in your free time. We truly recommend you to visit our friends on BestFreeSlots, if you would like to try some new and entertaining thing to do when relaxing. It’s definitely worth your visit. Under the optimal conditions, the molecular weight of the PLLA could increase from 62,100 g/mol to 126,000 g/mol by the triphenyl phosphite. The chemical structure were characterized by FTIR and 1H NMR and crytallinity …
Synthesis and crystal structure of a novel Salen-copper(II) complex

Abstract The Salen-copper(II) complex was synthesized by diffusion. Chemistry Online shows the structure which was characterized by single crystal X-ray diffraction. It crystallized in triclinic system,pB1spacegroup, with a=9.3441(18)Å, b=11.478(3)Å, c=12.320(3)Å, a=81.584(11)°, b=74.681(9)°, g=68.206(9), MolecularFormula: C23H19CuN5O8, M=397.86, V=1181.6(5)Å3, z=2, Dc=1.56Mg/m3, R1=0.0416, wR2=0.1236,I>[2s/(I)]. The crystal included a mononuclear complex [Cu(H2L)(DMF)] and a non-coordinated H2O molecular.
Keyword diaminomaleontrile;5-carboxylsalicylaldehyde;crystal structure.
Synthesis and Crystal Structure of a New Salen-Copper (II) Complex
Li Lijun, Yang Dongjie, Gao Yansu
(College of Chemistry and Environmental Science, Hebei University, Baoding, Hebei 071002, China )
Summary diffusion were synthesized Salen- copper (Ⅱ) mononuclear complexes [a Cu (H 2 L) (of DMF)] . H 2 0 [H . 4 L] = N, N’-bis (5-carboxy-salicylaldehyde) – Diaminomaleonitrile. The complexes were characterized by X-ray single crystal diffraction. The complexes belong to triclinic system, …
Adsorption and competitive adsorption of heavy metal ions lead, cadmium and copper on d-manganese oxide

Chemistry Magazine Online gives you another post with interesting chemistry news articles. Abstracts d- MnO2 was prepared with hydrothermal treatment, and its crystalline structure was verified with X-ray diffraction spectroscopy. FTIR showed there were copious hydroxyl groups on the surface of d-MnO2 for the adsorption. The adsorption capacity of three kinds of heavy metal ions Pb2+, Cd2+ and Cu2+ on the d-MnO2 was all increased with the increase of ion concentration and the increase of pH. The adsorption isotherms of Pb2+ exhibited good correlation with Freundlich equations, Langmuir equations and Temkin equations, whereas equilibrium data of Cu2+ was best fitted to Freundlich isotherm, and Langmuir isotherm was best for Cd2+. Adsorption thermodynamics indicates that as the temperature in the range of 298.15 ~ 323.15K, all the adsorptions were endothermic, spontaneous reaction. Therefore, increasing temperature is good for the …
A new biflavonoid from Selaginella uncinata (Desv.) Spring

Abstract A new biflavonoid, 5,7,4′,5”-tetrahydroxy-7”-metroxy-[3-O-4”’] biflavone along with four known biflavonoids and one known phenolic acid was isolated from the herb of Selaginella uncinata(Desv.) Spring. Their structures were elucidated by spectroscopic methods.
Keywords Selaginella uncinata;5,7,4′,5”-tetrahydroxy-7”-metroxy-[3-O-4”’] biflavone
-
INTRODUCTION
Selaginella uncinata(Desv.) Spring as a medicinal plant is used to treat acute infectious jaundice hepatitis, cholecystitis, enteritis and so on.In previous chemistry news articles about investigation of the plant, two kinds of chromone glycoside (uncinoside A and uncinoside B) have been separated from S. uncinata , which have good anti-tumor and anti-viral activity[1]. Our preliminary study found that ethyl acetate extract exhibited good anti-HSV-1 virus and Cox B3 virus activity [2]. Through activity-guided fractionation, we isolated a new biflavonoid, named 5,7,4′,5”-tetrahydroxy-7”- metroxy-[3-O-4”’] biflavone(IV), together with five known compounds:7,4′,7′,4”’– tetra-O-methylamento-flavone(I)[3],7, 7”, 4”’-trim-O-methyl amentoflavone(II)[4],7, 7”-di-O-methylamentoflavone(III)
Purification and analysis of lectin in ginkgo biloba seeds

The Sepharose CL-6B was used as an affinity chromatography column to purify and analyze the lectin in ginkgo biloba seeds. Chemistry in the news explains the coagulation experiment and comparison with the lectins present in ricinus and in seeds of bitter gourd explained that lectin in ginkgo biloba seeds was a protein with Only one subunit, which was similar to the galactose-specific recognition B chain in ricinus. The method adopted in this study is available for purifying the lectins from different plants.
Keywords Ginkgo biloba; Lectin; Purification and analysis
Purification and Analysis of Ginkgo Lectin
Xu Peng Peng Li Linzi Wang Guizhen Yan Haibo Li Renqiang (Department of Biotechnology, Jinan University, Guangzhou 510632, China )
Abstract The use of chromatography mediaSepharose CL-6Bcontains a lotB-chemical properties galactoside bond ligand, its purification affinity chromatography and analyzed ginkgo lectin. By comparing coagulation experiments with lectins in ramie and balsam pear, it was shown …
Kinetic and thermodynamic studies on the adsorption of Ni 2+ onto chitosan-aluminium oxide composite material

Abstract Kinetics’ and Thermodynamics of The Adsorption of of Ni 2+ Onto Chitosan-Aluminum Composite Material’s WAS Studied oxide. The Under Experimental Conditions, s Indicated Kinetic Adsorption of that of Ni 2+ Onto Aluminum oxide-Chitosan Composite Material’s Conforms to pseudo-SECOND-Order Equation And the apparent adsorption activation energy is 28.62 kJ/mol.In the experimental temperature, Chemistry Magazine Online and thermodynamic studies indicated that adsorption of Ni 2+ onto chitosan-aluminium oxide composite material conforms to Langmuir adsorption isotherm equation. Enthalpy of adsorption ( D H) value is 15.93 kJ/mol and entropy of adsorption( D S)value is 95.43J/(mol· K).Gibbs free energy ( DG) decreases with increasing temperature.
Keywords chitosan; composite material; adsorption;
Adsorption Kinetics and Thermodynamics of Ni 2+ on Chitosan-Aluminum Oxide Composites
MA Zhi-guang, ZHANG Fang, GENG Na, LIU Su-wen, LIU Pan
(College of Chemistry and Environmental Science, Hebei University, Baoding, Hebei 071002, China)
Abstract The kinetics and thermodynamics of Ni 2+ adsorption on …
Kinetics and mechanism of ruthenium(III) catalyzed oxidation of isobutyl alcohol by cerium(IV) in acid medium
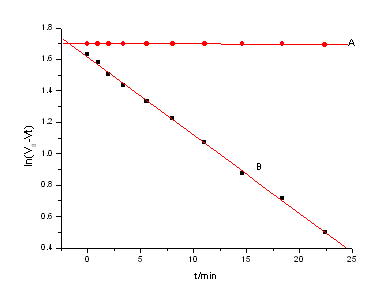
Abstract for news about chemistry – The kinetics and mechanism of ruthenium(III) catalyzed oxidation of isobutyl alcohol(IBA) by cerium(IV) in sulfuric acid media has been investigated by titrimetric technique of redox in the temperature range of 298-313K. It is found that the reaction is first-order with respect to Ce(IV) and Ru(III), and a positive fractional order with respect to IBA. It is found that the pseudo first-order ([IBA]>>[Ce(IV)]>>[Ru(III)]) rate constant kobs increases with the increase of [H+] but decreases with the increase of [HSO4–]. Under the protection of nitrogen, the reaction system can not initiate polymerization of acrylonitrile, indicating generation of free radicals. On the basis of the experimental results, a suitable mechanism has been proposed. The rate constants of the rate determining step together with the activation parameters were evaluated.
Keywords Ruthenium(III) ion; Cerium(IV) ion; Isobutyl alcohol(IBA); Catalytic Oxidization; Kinetics and mechanism
- INTRODUCTION
