Before we start, I like to mention my latest discovery on the internet – chemical slot games. I stumbled on them on accident while researching something and I was so impressed and entertained after playing them for a couple of hours that I had to share it with you. Just visit this site to get a free no deposit bonus and play them without risking money. And now let’s talk about Cure Kinetics.
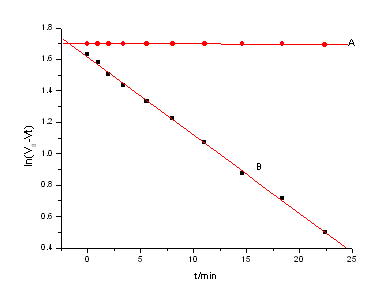
Abstract Cure kinetics of epoxy resin E51/MP/methyltetrahydrophthalic anhydride (MeTHPA)/ 2-ethyl-4-methyl-imidazole (2,4-EMI) flame retardant composite were studied using the dynamical tortional vibration method. The influence of MP loadings on The cure showed has improved studied were well. The results showed that the rate of cure reactions depended distinctly on the cure temperature. At low MP loadings, the gelation time t g for the systems did Not change obviously while at higher MP loading (higher than 20 phr) t …