Abstract The Salen-copper(II) complex was synthesized by diffusion. Chemistry Online shows the structure which was characterized by single crystal X-ray diffraction. It crystallized in triclinic system,pB1spacegroup, with a=9.3441(18)Å, b=11.478(3)Å, c=12.320(3)Å, a=81.584(11)°, b=74.681(9)°, g=68.206(9), MolecularFormula: C23H19CuN5O8, M=397.86, V=1181.6(5)Å3, z=2, Dc=1.56Mg/m3, R1=0.0416, wR2=0.1236,I>[2s/(I)]. The crystal included a mononuclear complex [Cu(H2L)(DMF)] and a non-coordinated H2O molecular.
Keyword diaminomaleontrile;5-carboxylsalicylaldehyde;crystal structure.
Synthesis and Crystal Structure of a New Salen-Copper (II) Complex
Li Lijun, Yang Dongjie, Gao Yansu
(College of Chemistry and Environmental Science, Hebei University, Baoding, Hebei 071002, China )
Summary diffusion were synthesized Salen- copper (Ⅱ) mononuclear complexes [a Cu (H 2 L) (of DMF)] . H 2 0 [H . 4 L] = N, N’-bis (5-carboxy-salicylaldehyde) – Diaminomaleonitrile. The complexes were characterized by X-ray single crystal diffraction. The complexes belong to triclinic system, P-1 space group, unit cell parameters: a=9.3441(18) Å , b=11.478(3) Å , c=12.320(3) Å , a =81.584(11)°, b = 74.681(9)°, g =68.206 (9) °, Molecular Formula: C 23 H 19 CuN 5 O 8 , M=397.86 V=1181.6(5) Å 3 z= 2 , Dc=1.56 Mg/m 3 R 1 = 0.0416, wR 2 = 0.1236I> [2 s/ (I)]. The crystal consists of a mononuclear complex [Cu(H 2 L)(DMF)] and an H 2 O guest molecule.
Keywords diaminomaleonitrile; 5- carboxysalicylaldehyde; crystal structure.
1 Introduction
Salen complexes play an important role in the transition metal coordination chemistry due to their ease of preparation and structural diversity, and they are one of the important stereochemical models. Ligands and their complexes have made great progress in the fields of catalysts, medicine and pesticides, corrosion inhibitors, development and research of new materials, organic synthesis, and analytical chemistry, and have been widely used [1 -4] . Salicylaldehyde Schiff bases and their complexes have attracted the attention of scientific workers due to their excellent antiviral, antitumor, bactericidal, and antifungal properties . [5-8] In this paper, N,N’ -bis (5- carboxysalicylaldehyde ) -diaminomethylenedicarboxylate was used as a ligand to synthesize N,N’ -di (5- carboxysalicylaldehyde ) by the reactant diffusion method . The diaminomalonitrile complex single crystal was characterized by X-ray .
2 Experimental
2.1 Experimental reagents and apparatus Parahydroxybenzoic acid (Fluka); CuCl 2 ·2H 2 O (Beijing Chemical Plant); diaminomaleonitrile (ACROS Corporation); other organic reagents are analytical reagents, Use directly. Nicolet 380 infrared spectrometer (400-4000 cm -1 , KBr tablet), Bruker 500 (500 MHz) NMR spectrometer. 2.2 Synthesis of ligands and complexes P -hydroxybenzoic acid 25g (60mmol) and NaOH 50g were placed in a 500ml three-necked flask, dissolved in 200ml of distilled water with nitrogen and then heated to 70°C under nitrogen and added in batches. CHCl 3 30 ml (370 mmol). The reaction mixture was stirred and refluxed at 70° C. for 8 h to cool, and then acidified with concentrated hydrochloric acid to pH=2 to produce a large amount of white precipitate which was filtered by suction to obtain a white solid. The solid was washed with water and dried to give crude 5- carboxysalicylaldehyde. The unseparated crude 5-carboxysalicylaldehyde (calculated as 10%) was dissolved in 100 ml of anhydrous methanol, and 2.0 g of diaminomaleonitrile (18.5 mmol) in 50 ml of anhydrous methanol was added dropwise. Hours, a yellow precipitate formed, which was isolated and dried to give 650 mg of product in 87% yield. 1 H-NMR (DMSO, ppm): d : 7.03 (d, 2H), 7.80 (d, 2H), 7.91 (s, 2H), 8.58 (d, 2H).
2.3 Complex [a Cu (H 2 L) (of DMF)] . H 2 single crystals prepared 0
to 3.5 mg of (0.04 mmol) of CuCl 2 · 2H 2 O were dissolved in 2ml of water was added dropwise to the lower elongated tube, carefully intermediate Add 1 ml DMF :H 2 O=1:1 mixture blank, add 8.8 mg (0.002 mmol) of ligand L dissolved in 2 ml DMF, and carefully added to the upper layer of the blank, let stand for a week, there is a red metal Shiny flake crystals.
2.4 Determination of single crystal structure of complexes Single red crystals of 0.32×0.32×0.06 mm 3 were selected and wrapped with epoxy resin and fixed at the top of the glass filaments for X-ray diffraction analysis. Single crystal diffraction data were collected on a Rigaku Saturn 724 CCD diffractometer using graphite monochromated Mo K a radiation ( l = 0.71073 Å ) and diffraction data collected at 293 (2) K. Scanning range: 2.41°< q <27.48°, -11<h<12,-14 < k <14,-15 <l < 15. Here you can find another post with similar content about current chemistry news.
Table 1. Crystal Data and Structure Refinement
| subject | data |
| Formula | C 23 H 19 CuN 5 O 8 |
| fw (g.mol-1) | 556.97 |
| Crystal system | Triclinic |
| Space group | pB1 |
| Unit cell dimensions |
a =9.3441(18)Åa=81.584 (11)° b=11.478(3)Åb =74.681(10)° c=12.320(3)Åg=68.206 (9)° |
| Flight [ Å 3 ] | 1181.6(5) |
| FROM | 2 |
| Dc(mg/m-3) | 1.565 |
| m [mm-1] | 1.377 |
| Absorption coefficient | 0.984 mm-1 |
| Crystal size (mm) | 0.32×0.32×0.06 |
| q range for data collection | 2.41 to 27.48 |
| Index ranges | -11<h<12,-14<k<14 ,-15<l< 15 |
| Reflections collected | 5336 |
| Independent reflections | 5336 |
| Data/restraints/parameters | 5536 / 0 / 374 |
| Final R indices [I>2s/(I) ] | R1 = 0.0416 wR2 = 0.1236 |
| Largest diff. peak and hole | 0.983 and -0.785 eÅ-3 |
Table 2. Selected bond lengths(Å) and angles(°) for compound
|
Cu (1) -O (4) 1.9013 (17) |
O (4) -Cu (1) -O (3) 89.41 (7) |
|
Cu (1) -O (3) 1.9095 (18) |
O (4) -Cu (1) -O (7) 94.00 (8) |
|
Cu (1) -N (1) 1.9557 (19) |
O (4) -Cu (1) -N (1) 173.83 (8) |
|
Cu (1) -N (3) 1.965 (2) |
O (3) -Cu (1) -N (3) 172.23 (8) |
|
Cu (1) -O (7) 2.376 (2) |
O (4) -Cu (1) -N (3) 92.72 (8) |
|
C (1) -O (1) 1.269 (3) |
N (1) -Cu (1) -N (3) 83.41 (8) |

Fig. 1 ORTEP diagram of complexes and atomic numbering
3 Results and discussion The structure of the complexes shows that the coordination number of the copper atom is 5, and its coordination atoms include two imine nitrogen atoms (N1, N3), two hydroxyl oxygen atoms (O3, O4) and a DMF. The oxygen atom (07). The geometric environment around the copper atom can be seen as a quadratic pyramid, where N1, N3, O3, O4 form the quadrilateral base of a quadratic pyramid, copper atoms are at the center of the bottom face, Cu(1)-N(1), Cu(1) The bond lengths of -O(3), Cu(1)-O(4), and Cu(1)-N(3) are 1.9095(18) Å , 1.9013(17) Å , 1.9557(19) Å , 1.965 ( 2) Å. Cu(1)-N(1) is almost perpendicular to Cu(1)-O(3), and the bond angle of O(3)-Cu(1)-N(1) is 93.81(8)°. O7 is located in the quadrangular pyramid. The vertex position of Cu(1)-O(7) is 2.376(2) , and Cu(1)-O(7) is perpendicular to the bottom of the quadrangular pyramid O(4)-Cu(1)-O(3). The bond angles of O(3)-Cu(1)-O(7) are 89.41(7)° and 91.55(8)°, respectively. The complex monomer forms a two-dimensional supramolecular system through intermolecular forces. The carboxyl groups at both ends of the ligands are connected to each other through hydrogen bonding to form a one-dimensional long chain. The interaction between different molecules is different. A dimer is formed between two complex molecules.
4 Conclusions A novel Salen complex was synthesized by the diffusion method. The single crystal structure was determined by X-ray diffraction. Analysis shows that the crystal includes a mononuclear complex and an H2O molecule.
Figure 2Crystal structure along the b-axis
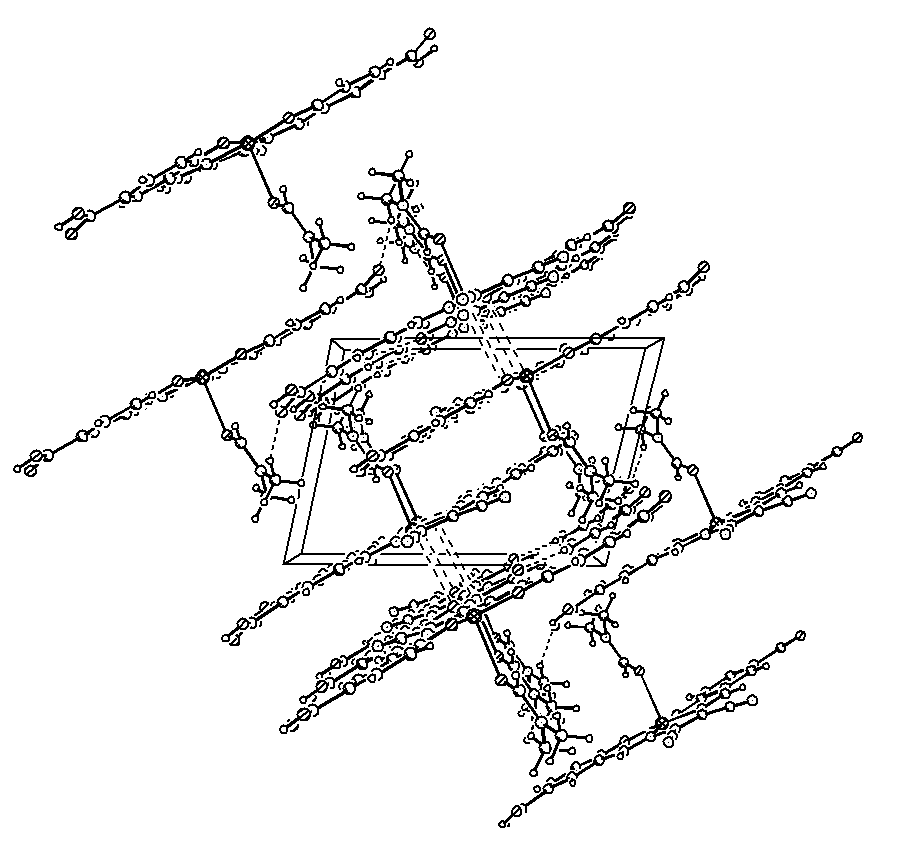
REFERENCES [1]Larrow JF, Jacobsen EN, Gao Y, et al. J.Org.Chem , 1994 , 59(7) : 1939-1942 [2] Qiu Min, Liu Guosheng, Yao Xiaoquan, et al. Chinese Journal of Catalysis, 2001 , 22 (1) : 77-80 [3] Jones RD , Budge JR , Jr PE , et al . Chem . Rev . , 1979 , 79 (2) : 139-179 [4] Wang cited true, in Wenjin, Gang like. Chinese Journal of rare earths, 2000 , 18(1) : 71-73 [5] Bi Siwei, Liu Shuxiang. Chinese Journal of Inorganic Chemistry, 1996, 12(4): 423-426 [6] Bi Siwei McGowan Qing , Tian Jun Lian , and so on. Applied Chemistry, 1995, 12 (6): 13-16
[7] Wang Zhangfeng , Yang Guangming , Xu Jingying , and the like. Applied Chemistry, 1997, 14(3): 29-32
[8] Wang Jianhua, Lei Wen, Wang Yuanliang. Chemical Bulletin, 2002 , 65: 1-7
For any information you need, you can always contact us.

