Luo Juntao , Sun Weimin , Huang Wenqiang , Wu Qiang , Zhu Xiaoxia a,b
The State Key Laboratory of Functional Polymer Materials for Adsorption and Separation, Institute of Polymer Chemistry, Nankai University, Tianjin 300071, China; Department of Chemistry, Université de Montréal, Montréal, Quebec,H3C 3J7, Canada )
AbstractAn improved preparation of 2-polystyrylsulfonamidoethanol resin and its primary application as a polymer support in the synthesis of substituted hydantoin and isoxazoline were reported. A solvent-free synthetic procedure for preparation of 2-polystyrylsulfonamidoethanol resin was optimized by the use of a diffuse reflectance IR spectroscopy technique. Polystyrylsulfonyl chloride () was reacted with aminoethanol to yield the 2-polysyrylsulfoamidoethanol Resin . Resin was then used as a polymer support in solid phase synthesis of 1-phenylhydantoin and 3–nitrophenyl-5-carboxylisoxazoline. The results show that 2-polystyrylsulfonamidoethanol resin is a suitable and useful polymer support.
1 INTRODUCTION
Combinatorial chemistry has become a powerful tool to generate large and diverse molecular libraries[ 1]. Solid-phase organic synthesis has played an important role in combinatorial chemistry. Further development of new linkers and tether groups that can accommodate various sequences of reactions will facilitate the preparation of new types of libraries[1]. The hydroxyl groups on the polymer supports are one type of the most useful linkers in solid phase organic syntheses [2], and benzyl alcohol linker is the most common anchor for the compounds bearing carboxylic acid groups. The use of only a small number of aliphatic hydroxyl groups on the supports has been reported. Ruhland[3] and Prien[4] used hydroxyethyl-polystyrene as a support in solid phase synthesis. However, the cleavage of ester linker required a rigorous condition: the use of amines in the presence of a Lewis acid.
Akaji[5] attached 4-(1′,1′-dimethyl-1′- hydropropyl)phoxyacetyl (DHPP) group onto PEG-PS to give a polymer support with the sterically hindered DHPP linker. The attachment of the first residue onto the hydroxyl group of this resin by esterification requires stringent conditions, such as the use of the acid chloride, and the cleavage of ester anchor requires the use of 95% TFA. Another example is NovaSyn TG resin whose hydroxyl groups can couple with carboxyl compounds to form ester anchors, and the products can be released by saponification with aqueous 0.1 mol/L NaOH solution[6]. We report here a new type of functionalized polymer support bearing hydroxyl linker, i.e., a polystyrylsulfonamidoethanol resin () prepared by the reaction of polystyrylsulfonyl chloride resin ([7] with 2-aminoethanol. Resin can couple with carboxylic acids to form ester anchors, which can be cleaved by the use of both basic and acidic conditions.
2 RESULTS AND DISCUSSION
2.1 Preparation of 2-polystyrylsulfonamidoethanol (2)
The preparation of polystyrylsulfonamidoethanol was tedious as reported previously: polystyrylsulfonyl chloride resin was refluxed with aminoethanol in dichloromethane for 18 h [8]; or polystyrylsulfonyl chloride resin was reacted with aminoethanol in THF at room temperature for 24 h[9]. We used the diffuse reflectance IR spectroscopic technique to monitor and to optimize the preparation of 2-polystyrylsulfonamidoethanol (). Here you can find the most interesting chemistry news articles.
The diffuse reflectance IR spectroscopy technique has its advantages: ease of operation, use of small amount of sample, short of analytical time and good reproducibility of data[10,11]. The polystyrylsulfonamidoethanol resin () was synthesized by the reaction of polystyrylsulfonyl chloride resin () with 2-aminoethanol in the absence or presence of solvents and/or catalysts (Scheme 1). The reaction was monitored by the diffuse reflectance IR spectra. In the process of reaction the absorbance of benzene ring on the polystyrene backbone at 1492 cm-1 is constant and can act as an internal standard, while the absorbance at 1377 cm-1 (the characteristic absorbance of sulfonylchloride group) changes. The ratio of the intensity of the absorbance band at 1377 cm-1 to that of the absorbance band at 1492 cm-1 was used as a qualitative index to the reaction progress. The lower the ratio is, the more complete the reaction is. Table 1 lists the results of the reaction of Resin with aminoethanol in the absence or presence of solvents (HO or EtN) and catalysts (EtN or pyridine) at 60 °C for 4 h. It can be seen from Table 1 that Condition A provides the best result in sulfonamidation, i. e., the solvent-free procedure is the most favorable to the sulfonamidation of sulfonylchloride resin with aminoethanol. To optimize the reaction time the solvent-free reaction was followed with the IR technique as a function of time at 60 °C (Figure 1). Figure 1 shows the disappearance of the absorbance band at 1377 cm-1 after a time of 30 min indicating the completion of the sulfonamidation. To sum up the above results the optimal condition of sulfonamidation of Resin with aminoethanol is: polystyrylsulfonylchloride Resin and aminoethanol, 60 °C, 30 min. This procedure is much simpler and more effective than that previously reported in the literatures[8,9]. The loading of hydroxyl group on Resin was determined to be 1.92 mmol/g by element analysis of nitrogen. The conversion of sulfonylchloride group on Resin to sulfonamidoethanol group on resin is 94%.

Fig.1 The diffuse reflection IR spectra of sulfonylchloride Resin with aminoethanol varying with the reaction time
Table 1 The effects of solvents and catalysts on the sulfonamidation of sulfonylchloride Resin 1 with aminoethanol (60 °C, 4 h)
| Reaction condition | |||||
| Solvent | Nil | Et | |||
| Catalyst | Nil | Nil | Et | Pyridine | Nil |
| 1377/I1492 | 0.023 | 0.200 | 0.054 | 0.073 | 0.035 |
2.2 Application of Resin 2 in solid phase synthesis of 1-phenyl-hydantoin and isoxazoline
Many of the compounds derived from heterocyclic structures have biological activities. Therefore, these compounds have received special attention in combinatorial chemistry[12,13]. Among them hydantoins and isoxazolines are the most interesting ones[14,15] since they are not only potential pharmaceutical leads, but also precursors to useful intermediates[16]. Hanessian[14b] used Merrifield resin as a support in the synthesis of hydantoins. Cheng [15b] prepared D -isoxazolines on Wang’s resin. Shanker[15a] reported the synthesis of isoxazolines from 2-chlorotrityl resin-supported aldoximes. Our results in this report demonstrate that 2-polystyrylsulfonamidoethanol resin is a suitable and useful support in the solid phase synthesis of hydantoin, isoxazoline and related compounds.
Scheme 1
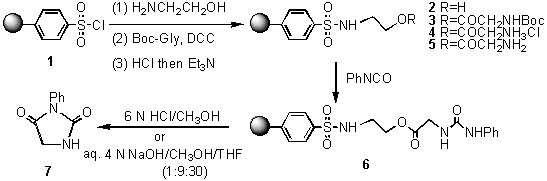
Application of Resin 2 in synthesis of 1-phenyl-hydantoin is showed in Scheme 1. The general DCC/DMAP coupling procedure can be used to attach Boc-protected glycin onto Resin 2 to afford Resin 3. After blocked the unreacted hydroxyl groups with benzoyl chloride/pyridine, deprotected the Boc group with 4mol/LHCl in dry 1,4-dioxane, and neutralized with EtN, Resin 5 with free amino groups was obtained. Polymer-supported urea (6) was prepared by the treatment of Resin 5 with phenyl isocyanate under nitrogen at ambient temperature and then cleaved with 6mol/Laqueous HCl to give hydantoin in an overall yield of 17% from Resin 3 (an isolated and crystallized yield). Resin 6 was also cleaved with a mixture of THF/MeOH/4mol/LNaOH (30:9:1 in volume) to obtain the same hydantoin product in an overall yield of 20% (an isolated and crystallized yield).
Scheme 2

The solid-phase synthesis of isoxazolines was carried out on Resin . As shown in Scheme 2, the hydroxyl group on Resin was reacted with acryloyl chloride in the presence of EtN to yield polymer-supported acrylate (Resin ). Resin was reacted with bromo–nitrobenzaldoxime () by a [3+2] cycloaddition to give the isoxazoline resin (10). An aqueous 6mol/LHCl solution was used to cleave the isoxazoline resin to obtain 3–nitrophenyl-5-carboxylisoxazoline (11) in an overall yield of 36% (an isolated and crystallized yield).
In summary, polystyrylsulfonamido ethanol () was prepared in optimization by the solvent-free reaction of polystyrylsulfonyl chloride with aminoethanol. Resin was found to be a useful polymer support for solid-phase syntheses of hydantoin and isoxazoline in acceptable yields. The polymer supported intermediates can be cleaved with both acid and base. The use of Resin in other solid-phase organic syntheses is being investigated.
3 EXPERIMENTAL
3.1 Preparation of 2-polystyrylsulfonamidoethanol (2)
To Resin (0.5 g, 2.15 mmol Cl/g) was added 2-aminoethanol (5 mL). The suspension was shaken at 60°C for 30 min in a mechanical shaker. After finished the reaction, the resin was filtered out and washed successively with HO, 2% aqueous HCl, and then HO to a neutral state, then with EtOH and EtO, and dried at 40 ° C under vacuum over P to afford Resin : N: 2.69%, corresponding to the loading of 1.92 mmol OH/g.
3.2 Synthesis of hydantoin on Resin 2
Attachment of Boc-glycin onto Resin : Resin (0.2 g) was swollen in DMF (5 mL) for 30 min. To the suspension were added Boc-Gly (0.18 g), DCC (0.2 g), DMAP (0.12 g). The mixture was mechanically shaken at 30 °C for 4 h. The resin was filtered out and washed in succession with DMF, DCM, DMF, EtOH, and EtO, and then dried over P at 40 °C overnight to give Resin
The conventional procedures were used to block the unreacted hydroxyl groups, to remove Boc protection group, and to neutralize hydrochloride: Resin was treated with benzoyl chloride/pyridine in DCM to block the unreacted hydroxyl groups. Resin was then treated with 4 N HCl in dry 1,4-dioxane to form Boc-deprotected Resin . Resin was neutralized with 5% EtN in DCM to afford Resin with free amino groups.
Preparation of polymer-supported urea (): Resin (0.2 g) was suspended in DMF (10 mL). To the suspension was added phenyl isocyanate (5 equivalents based on the loading of free amino group). The mixture was shaken under nitrogen at ambient temperature for 8 h. The resin was filtered out and washed in succession with DMF, DCM, DMF, EtOH, and EtO, and then dried over P at 40 °C under vacuum overnight to give urea resin ().
Preparation of hydantoin:
Acid-cleavage procedure: To the urea resin (, 0.2 g) was added aqueous 6 mol/L HCl (2 mL). The mixture was heated to 80 °C and maintained for 6 h. Then 1 mL MeOH was added to the mixture, which was refluxed for another 20 min. The resin was filtered out and washed twice with HO. The combined aqueous phase was neutralized with aqueous sodium carbonate, and concentrated. DCM (20 mL) was added to the residue to extract the product. After removed the inorganic salts by filtration, the concentrated solution was crystallized. The colorless and needle crystalline hydantoin (11 mg) was obtained in an overall yield of 17%, m.p.: 173°C.
Base-cleavage procedure: To the urea resin (, 0.15 g) was added 3.75 mL of a mixture of THF/MeOH/4mol/L NaOH (30:9:1 in volume). The mixture was allowed to stand at room temperature for 15 min. The resin was filtered out and washed twice with HO. The combined aqueous phase was neutralized with dilute HCl solution, and concentrated. DCM (20mL) was added into the residue to abstract the product into the oil phase. After removed the inorganic salts by filtration the concentrated solution was crystallized. The colorless and needle crystalline hydantoin (9.5 mg) was obtained in an overall yield of 20 %.
3.3 Synthesis of isoxazoline on Resin 2
Preparation of acrylate resin (): Resin (5 ) was swollen in DMF (50 mL) for 30 minutes. To the suspension were added acryloyl chloride (4.67 mL) and EtN (1.77 mL). The mixture was mechanically shaken at 25 °C for 2 h. The resin was filtered and washed twice each with DMF, DCM, EtOH and EtO, and then dried over P at 40 °C overnight to give the acrylate Resin (8)
Preparatiom of polymer-supported isoxazoline: The acrylate resin (0.5 ) was suspended in DCM (5 mL) for 30 minutes. To the suspension was added brominated -nitrobenzyl aldoxime (, 1.338 ). After EtN (0.016 mL) was added dropwise into the mixture, the system was allowed to stand at 25 °C for 24 h under stirring. The resin was filtered and thoroughly washed with DCM, DMF, EtOH and EtO, followed by drying over P at 40 °C overnight to give isoxazoline Resin 10
Cleavage of isoxazoline from Resin 10: To Resin 10 (0.5 ) was added aqueous 6 mol/LHCl solution (5 mL). The mixture was stirred at 60 °C for 8 h. The resin was filtered out and washed with distilled water twice. The combined aqueous phase was evaporated using a rotating evaporator. 3–Nitrophenyl-5-caboxylic acid-isoxazoline was crystallized (overall yield: 36%): M.P: 179~180.5 ° C; H NMR (ppm, CDCl): 3.6-3.8 (m, 2H), 5.2 (t,1H), 7.8-8.4 (m, 4Harom), 13.3 (s,1H).
