Abstract about interesting chemistry news articles for adsorptive stripping method for the determination of resorcinol on multi-walled carbon nanotube modified glassy carbon electrode (MWNT-GCE) was developed. Cyclic voltammetry was used in the study of the electrochemical behavior of resorcinol on MWNT-GCE. The sharp peak of resorcinol appeared at 0.6 V (vs. Ag/AgCl) with the differential pulse voltammetry (DPV). The experimental parameters were optimized. Anodic peak currents were linear over the resorcinol concentrations in the range of 5.5×10-5-3.3×10-3 g L-1 with a detection limit of 1.1×10-5 g L-1 and relative standard deviations of 3.30-6.94% for 7 determinations. The interference of organic and inorganic species on the voltammetric response was also tested. The proposed method was applied to the determination of resorcinol in tap water and simulative wastewater samples with an average recovery of 95.2-104.8%.
Keywords Determination; Carbon nanotube; Modified glassy carbon electrode; Differential pulse voltammetry; Resorcinol
-
INTRODUCTION
Resorcinol is an important industrial chemical material used widely in the fields of rubber, plastics, and organic synthesis industries, wood adhesives, fire retardants, and UV stabilizer, etc. It is derived from various resins and tannins but most commonly by fusing sodium hydroxide with meta-benzene-disulfuric acid. Global output of resorcinol in 2004 was about 41.83 million tons. This compound is a moderate toxic substance and easily soluble in water[1]. There is a growing need for developing highly sensitive, simple methods to detect resorcinol in the wastewater at a low level. Various technologies for the detection of resorcinol have been developed, such as high performance liquid chromatography (HPLC)[2,3], thin-layer chromatography[4], spectrophotometry[5,6], surface plasmon resonance[7], and biochemical methods, including enzyme[8], bioluminescence[9], biosensor[10]. However, sample pre-treatment processes involving separation, extraction, and adsorption are generally necessary, and these steps are time-consuming and complex.
The chemical modification of electrode (CME) is a field of growing interest in analytical chemistry. The modification of the electrode surface could promote reactivity and selectivity to the base sensor, leading to the development of electrodes for some specific applications[11,12]. It has been demonstrated that CME possesses distinct advantages over conventional electrode in many application areas including electrocatalysis and electrochemical sensors. One of the most important properties of the CME, which has been the subject of considerable study, is their ability to catalyze the oxidation or reduction of some compounds[13,14]. Carbon nanotube is of fullerene-related structures that consist of graphite cylinder closed at either end with cap containing pentagonal ring. It is promising as immobilization substance because of its significant mechanical strength, high surface area, excellent electrical conductivity and good chemical stability. The main advantages of a nanoparticle-modified electrode compared to a macroelectrode are high effective surface area, mass transport, catalysis and control over local microenvironment[15]. In essence the small dimension of nanoparticle brings about convergent rather than linear diffusion, resulting in a higher rate of mass transport to the electrode surface. However, this can modify the appearance of the voltammetric signal due to the increased mass transport overpotential. In view of the excellent properties of carbon nanotube, it has been used to modify different electrodes for determination of chemical and biological species[16].
Not many electrochemical studies of resorcinol have been reported. Nasr et al.[17] and Robson T. S. Oliveira et al.[18] reported the electrochemical oxidation of resorcinol on boron-doped diamond anodes. Cyclic voltammetric study showed that the current density of resorcinol was smaller and no reverse peak was observed. Wang group[19] reported the method of simultaneous determination of hydroquinone, resorcinol, and catechol by voltammetry with single-walled carbon nanotube (SWNT) modified glassy carbon electrode. The detection limit of resorcinol was 3.3×10-5 g L-1. Each of these studies has reported the electrochemical behavior or the determination of dihydroxybenzene isomers, but so far there is no report on the analysis of resorcinol by voltammetry with multi-walled carbon nanotube (MWNT) modified glassy carbon electrode.
This work investigated a new method for the determination of resorcinol by differential pulse voltammetry (DPV) using a glassy carbon electrode modified by multi-walled carbon nanotube, which promoted better electron transfer between the electrode surface and electroactive species in solution. The modified electrode exhibited a remarkable accumulation effect for resorcinol and high sensitivity. The proposed method also displayed good resistance to the interference from some organic and inorganic compounds. -
EXPERIMENTAL
2.1. Apparatus
The voltammograms were obtained by using a MEC-12B microcomputer-controlled multimode electrochemical analyzer with an ATA-B rotating disk electrode and a three-electrode set-up (Jiangfen Electroanalytical Instrumental Ltd., Jiangyan, China). The experiments were carried in a three-electrode cell containing a glassy carbon electrode (GCE, 3 mm in diameter) as working electrode, an Ag/AgCl electrode with saturated KCl as reference electrode, and a platinum wire as counter electrode. The software package of electrochemical analyzer system worked through a personal computer with data acquisition routine. Repetitions of voltammetric measurements for each resorcinol concentration were automatically controlled with a computer program in order to achieve good statistics of the measurements. A KQ-50E ultrasonic cleaner (Kunshan Ultrasonic Instrument Company Ltd., Shanghai, China) was employed during the electrode preparation process. SiC sandpaper was used to polish GCE.
Capillary electrophoresis (CE) determination was performed by an Agilent 3D CE system with air-cooling and a diode array detector (Agilent Technologies, Waldbronn, Germany) to validate the proposed electrochemical method. A 48.5 cm (40.0 cm to the detector) × 50 mm i.d. uncoated fused-silica capillary (Agilent) was utilized. During the determination process, capillary temperature and applied voltage were set at 20 ℃, 15 kV respectively. UV detection was carried at 280 nm.
2.2. Reagents and solutions
An aqueous stock standard solution containing 1.1 g L-1 resorcinol was prepared by dissolving 0.11 g resorcinol in 100 mL water. Suspension of 1.0 g L-1 multi-walled carbon nanotube was prepared by adding 50 mg MWNT(30~50 nm) into 50 mL water and sonicated for 20 min. 0.2 mol L-1 sodium hydroxide (NaOH) was prepared to activate electrode. pH=7.0 buffer solution of KCl, Na2HPO4, KH2PO4 was prepared by mixing 50 mL 0.2 mol L-1 KCl solution, 12.5 mL 0.1 mol L-1 Na2HPO4 solution, and 25 mL 0.2 mol L-1 KH2PO4 solution, then diluted to 100 mL. The buffer solution was used throughout this study, unless stated otherwise.
All chemicals were of analytical-reagent grade. All solutions were prepared using 18.3 MW water obtained from a Mili-Q purification system.
2.3. Preparation of the modified electrode
The GCE was previously polished with a piece of SiC sandpaper, then thoroughly washed with doubly distilled water and anhydrous ethanol in the ultrasonic cleaner for 5 min, respectively, and dried in air. MWNT films were prepared by casting 50 mL aqueous dispersion of 1.0 g L-1 MWNT onto GCE, then dried under an infrared light. The modified electrode was activated by immersing in 0.2 mol L-1 NaOH and then cleaned electrochemically by cycling potential scans for five cycles between -1.5 to 1.5 V at a scan rate of 200 mV s-1 and accumulation potential of -1.5 V.
2.4. Analytical procedures
5 mL buffer solution (pH=7.0) was transferred into a dry sample cell and resorcinol standard stock solution was added with definite volume by a micropipette. The above solution was diluted to 10 mL with buffer solution and deoxidized by bubbling with extra pure nitrogen for about 15 min before electrochemical measurements. After a 15 s rest, the DPV method was applied from 0 V to 1.3 V to the modified GCE (versus Ag/AgCl reference electrode). The pulse amplitude, pulse width, accumulation time, accumulation potential was 50 mV, 50 ms, 60 s, -0.4 V respectively. The measured data were recorded by a workstation with 10 mV intervals.
Cyclic voltammetry (CV) method was applied for acquiring qualitative information about electrochemical reactions, the cyclic voltammograms were recorded by applying a scan over the potential range from 0 V to 1.3 V to the GCE (versus Ag/AgCl reference electrode). The accumulation time, accumulation potential, and scan rate were 60 s, -0.4 V, and 200 mV s-1, respectively. Data acquisition and treatment were performed with a workstation. -
RESULT AND DISCUSSION
3.1. Electrochemical response of resorcinol on WMNT modified glassy carbon electrode
The cyclic voltammograms of this system are shown in Fig. 1. Anodic peak increased obviously after addition of resorcinol and this result suggested that anodic peak of resorcinol was produced on GCE. The anodic peak potential was at 0.6 V on the GCE in pH=7.0 buffer solution. Only one anodic peak was observed and this result confirmed the irreversibility of the electrochemical reaction under investigated conditions.

Fig. 1 Cyclic voltammograms of resorcinol in pH=7.0 buffer solution.
a-blank solution, b-5.5×10-3 g L-1 resorcinol. The accumulation time, accumulation potential, and scan rate were 60 s, -0.4 V, and 200 mV s-1, respectively.
3.2. Influence of modified electrode
In our study, bare GCE, MWNT modified GCE, and sodium montmorillonite modified GCE were compared during the determination of resorcinol, respectively. The results were shown in Fig. 2. According to the sensitivity of each electrode, MWNT modified GCE obtained a best result.

Fig. 2 Comparison of different kinds of electrode. a – sodium montmorillonite modified GCE; b – MWNT modified GCE; c- bare electrode.
(Conditions: pH of buffer solution was 7.0. The accumulation time, accumulation potential, scan rate and the concentration of resorcinol were 60 s, -0.4 V, 200 mV s-1, and 5.5×10-3 g L-1, respectively.)
3.3. The choice of buffer solution and pH
It was important for the chemically modified electrode to choose the buffer solution and the pH of the buffer solution. A series of buffer solutions were tested. In 0.2 mol L-1 Na2CO3 + NaHCO3 (pH=9.5~11.5), NaOH + sodium citrate (pH=4.0~6.0), and 0.2 mol L-1 NaOH solution, no peak appeared. In KH2PO4+NaOH (pH=5.8~8.0), KH2PO4 + Na2HPO4 + KCl (pH=6.6~7.8), a cathodic peak appeared. The peak height and shape in KH2PO4 + Na2HPO4 + KCl were optimum, so we chose KH2PO4 + Na2HPO4 + KCl buffer solution for subsequent studies.
Effect of pH on peak current and peak potential of resorcinol was investigated in KH2PO4 + Na2HPO4 + KCl buffer solution in the pH range from 6.60 to 7.80. The results were shown in Fig. 3. The voltammetric peak potential for resorcinol became more negative with the increase of pH in a roughly linear manner. This suggested that the concentration of hydrogen ions affected the rate of reaction. The hydroxyl group dissociated gradually and shifted the peak to more negative values as pH increased. The H+ concentration affected the peak current of resorcinol obviously also. The peak current gave a maximum peak in pH=7.0 buffer solution. Thus, the buffer solution of pH=7.0 was selected as a suitable analytical medium in this study. At pH=7.0, concentrations of KH2PO4, Na2HPO4, and KCl were 0.05 mol L-1, 0.0125 mol L-1 and 0.1 mol L-1, respectively.

Fig. 3 Influence of pH of the KH2PO4 + Na2HPO4+ KCl buffer on peak current and peak potential.
The accumulation time, accumulation potential, and scan rate were 60 s, -0.4 V, and 200 mV s-1, respectively (5.5×10-3 g L-1 resorcinol)
3.4. Influence of accumulation time and potential
It should be mentioned that it was important to choose accumulation time and potential when adsorption studies were undertaken, since both parameters could influence the degree of adsorption of the resorcinol reaction. Bearing this in mind, the effect of accumulation time and potential on anodic peak current was investigated by CV.
The peak current increased with the accumulation time in the range from ca. 0 to 60 s. However, at about 60 s, further increase in the accumulation time couldn’t increase the amount of resorcinol at the electrode surface due to surface saturation, and the peak current remained constant.
The effect of accumulation potential was examined by using 5.5×10-3 g L-1 resorcinol at a constant time of accumulation of 60 s over the range from 0 to 0.7 V in pH 7.0 buffer solution. The results were shown in Fig. 4. The responses were obviously influenced by the accumulation potential. The optimal accumulation potential was 0.4 V, which was chosen for further studies.

Fig. 4 Effect of accumulation potential on peak current.
The accumulation time, accumulation potential, and scan rate were 60 s, -0.4 V, and 200 mV s-1, respectively (5.5×10-3 g L-1 resorcinol)
3.5. Reproducibility
The 5.5×10-5~3.3×10-3 g L-1 resorcinol was determined repeatedly with the same electrode for 7 times. The average R.S.D. was 3.99~8.94%. It indicated that the reproducibility of the MWNT modified GCE was remarkable. Voltammetric response of resorcinol (5.5×10-5–3.3×10-3 g L-1) on MWNT modified GCE was plotted in Fig. 5.
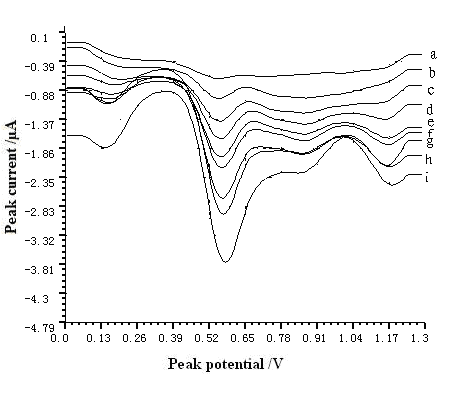
Fig. 5 Voltammetric response of resorcinol on MWNT modified GCE. The pH, The accumulation time, accumulation potential, and scan rate were 7.0, 60 s, -0.4 V, and 200 mV s-1, respectively. (a) 5.5×10-5 g L-1 (b) 2.2×10-4 g L-1 (c) 4.4×10-4 g L-1 (d) 6.6×10-4 g L-1 (e) 8.8×10-4 g L-1 (f)1.1×10-3 g L-1 (g) 1.65×10-3 g L-1 (h) 2.2×10-3 g L-1 (i) 3.3×10-3 g L-1
3.6. Linear range and detection limit
The peak current was proportional to the concentration of resorcinol in the range of 5.5×10-5~3.3×10-3 g L-1 with correlation coefficient of 0.9986. The linear regression equation was Ip = 6.20×10-3C + 0.12241, in which Ip and C indicated the peak current in mA and analyte concentration in 10-5 g L-1, respectively. The detection limit of the resorcinol in water was 1.1×10-5 g L-1. The voltammetric response of resorcinol was practically stable at least 1 h, with a maximum decrease less than 5.0% being quite satisfactory for analytical purposes.
3.7. Interferences
The interferences from some metal ions, anions and other organic compounds were examined with 5.5×10-3 g L-1 resorcinol. Measurements of the peak current for each solution were repeated three times and the average current values were obtained. If the presence of a compound altered the average current of resorcinol less than ±5%, then the compound was considered that did not cause interference. The results showed that 100 folds of Mg2+, Ca2+, K+, Na+, Mn2+, Cd2+, Cu2+, Br–, NO3–, SO42-, CO32-, PO43-, and 100 folds of phenol, methanol and ethanol had no obvious influence on the determination of resorcinol. However, 2 folds excess of ascorbic acid, o-nitrophenol and catechol caused the peak depression about 10%.
3.8. Practical analysis of samples
This method was applied to the determination of resorcinol in samples of simulative wastewater and tap water respectively. The simulative wastewater sample was constructed by adding resorcinol and co-existing components in tap water, which contained 5.0×10-4 g L-1 phenol, 5.0×10-4 g L-1 ethanol, 1.0×10-4mol L-1 NaCl, 1.0×10-4 mol L-1 Na2SO4 and different amount of resorcinol. The concentration of resorcinol was determined by the standard addition method.
The determination of simulative wastewater and tap water was carried respectively by CE method also to validate the proposed method. Results were listed in Table 1. The recoveries and CE method suggested that the proposed method was effective for the determination of resorcinol.
Table 1 Determination and results recovery of samples
| Sample | Resorcinol in samples (×10-4g L-1) |
Added (×10-4g L-1) |
Found by proposed method (×10-4g L-1) |
Recovery (%) |
Relative Standard Deviation (%) | Found by CE method (×10-4g L-1) |
| Tap water 1 | 0 | 5.0 | 4.76 | 95.2 | 4.74 | 4.80 |
| Tap water2 | 0 | 10.0 | 10.48 | 104.8 | 5.09 | 10.12 |
| Wastewater 1 | 3.8 | 5.0 | 8.68 | 98.6 | 3.18 | 8.60 |
| Wastewater 2 | 4.2 | 10.0 | 14.47 | 101.9 | 4.39 | 14.21 |
-
CONCLUSIONS
The proposed method was an effective method for the analysis of resorcinol. It was simple, accurate and precise, and time saving. The determination of resorcinol on glassy carbon electrode modified by carbon nanotube would be a choice method for resorcinol monitoring in wastewater.
REFERENCES
[1] Sangli A B, Kanabur V V, Environ. Ecol., 1998, 16 (3): 642-644.
[2] Cui H, Zhou J, Xu F, et al. Anal. Chim. Acta, 2004, 511 273-279.
[3] Wang L H, Kuo Y P. Chromatographia, 1999, 49: 208-211.
[4] Sharama O P, Bhat T K, Singh B. J. Chromatogr. A, 1998, 82 (1): 167-171.
[5] Khalaf K D, Hasan B A, Morales-Rubio A, et al. Talanta, 1994, 41 (4): 547-556.
[6] Onur F, Acar N. Int. J. Pharm., 1992, 78 (1-3): 89-91.
[7] Wright J D, Oliver J V. Sens. Actuators. B, 1998, 51 (1-3): 305-310.
[8] Bagirova N A, Shekhovtsova T N, van Huystee R B. Talanta, 2001, 55: 1151-1164.
[9] Metzger J, Reiss M, Hartmeier W. Biosens. Bioelectron., 1998, 13 (10): 1077-1082.
[10] Kudryasheva N, Kratasyuk V, Esimbekova E, et al. Field. Anal. Chem. Technol., 1998, 2 (5): 277-280.
[11] Davis J J, Coles R J, Hill H A O. J. Electroanal. Chem., 1997, 440: 279-282.
[12] Katz E, Willner I, Wang J. Electroanal., 2004, 16: 19-44.
[13] Britto P J, Santhanam K S V, Ajayan P M. Bioelectrochem. Bioenerg., 1996, 41: 121-125.
[14] Ozkan S A, Ozkan Y, Senturk Z. J. Pharm. Biomed. Anal, 1998, 17: 299-305.
[15] Davis J J, Green M L H, Hill H A O, et al. Inorg. Chim. Acta, 1998, 272: 261-266.
[16] Luo H X, Shi Z J, Li N Q, et al. Anal. Chem., 2001, 73: 915-920.
[17] Nasr B, Abdellatif G, Canizares P, et al. Environ. Sci. Technol., 2005, 39: 7234-7239.
[18] Oliveira R T S, Salazar-Banda G R, Santos M C, et al. Chemosphere, 2007, 66: 2152-2158.
[19] Wang Z H, Li S J, Lv Q Z. Sensors and Actuators B, 2007, 127: 420-425.

